Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (10): 2030-2037.doi: 10.12307/2025.224
Previous Articles Next Articles
Hydroxyapatite-graphene oxide composite coating promotes bone defect repair in rats
Dumanbieke·Amantai, He Huiyu, Han Xiangzhen
- Department of Prosthodontics, First Affiliated Hospital (Affiliated Stomatological Hospital) of Xinjiang Medical University, Xinjiang Uygur Autonomous Region Institute of Stomatology, Urumqi 830000, Xinjiang Uygur Autonomous Region, China
-
Received:2023-10-30Accepted:2024-01-20Online:2025-04-08Published:2024-08-21 -
Contact:Han Xiangzhen, Master, Attending physician, Department of Prosthodontics, First Affiliated Hospital (Affiliated Stomatological Hospital) of Xinjiang Medical University, Xinjiang Uygur Autonomous Region Institute of Stomatology, Urumqi 830000, Xinjiang Uygur Autonomous Region, China -
About author:Dumanbieke·Amantai, Master candidate, Department of Prosthodontics, First Affiliated Hospital (Affiliated Stomatological Hospital) of Xinjiang Medical University, Xinjiang Uygur Autonomous Region Institute of Stomatology, Urumqi 830000, Xinjiang Uygur Autonomous Region, China
CLC Number:
Cite this article
Dumanbieke·Amantai, He Huiyu, Han Xiangzhen. Hydroxyapatite-graphene oxide composite coating promotes bone defect repair in rats[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2030-2037.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
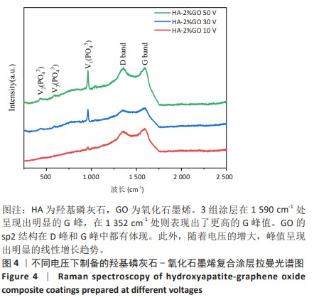
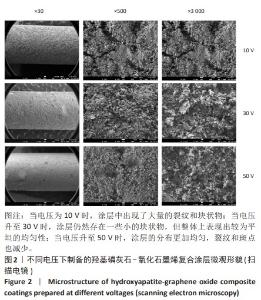
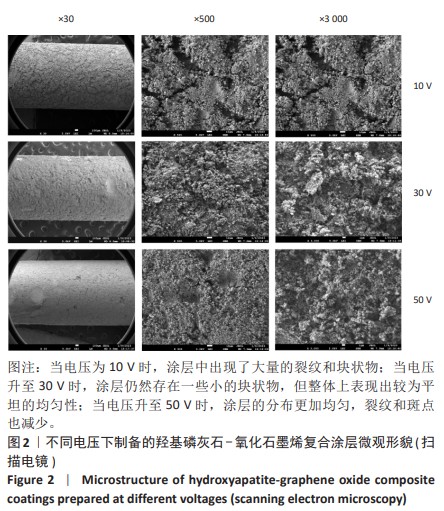
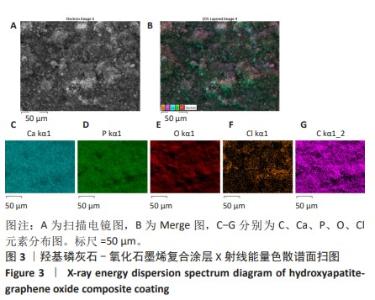
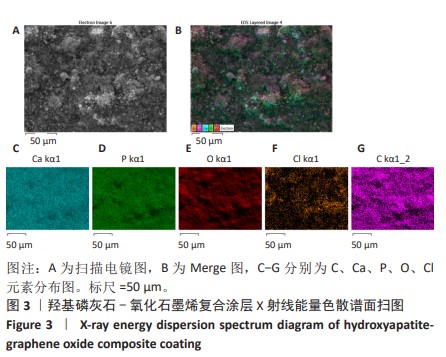
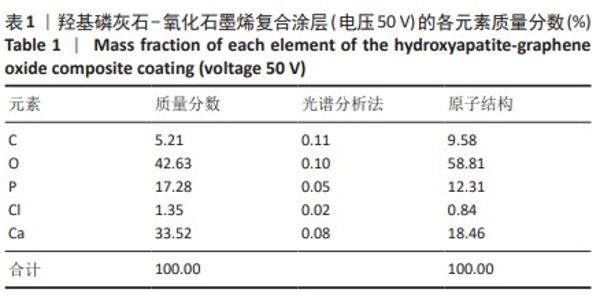
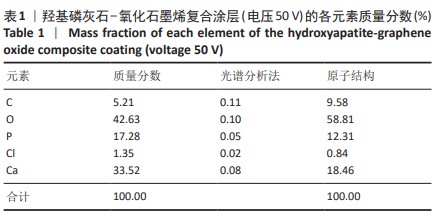
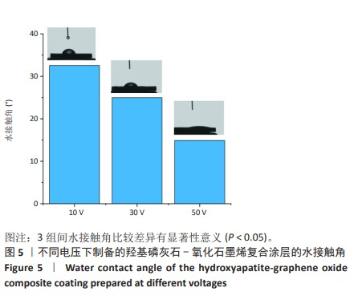
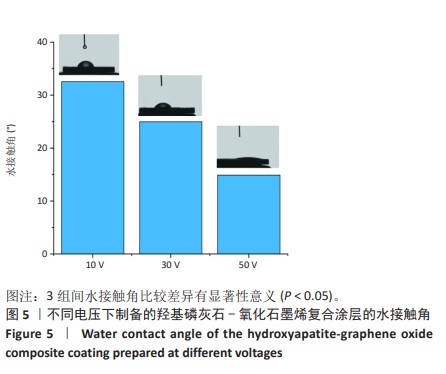
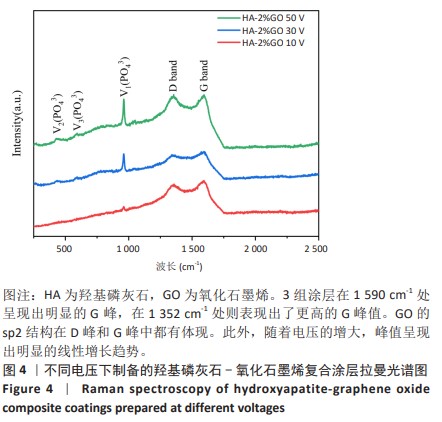
2.1.3 复合涂层拉曼光谱分析结果 从拉曼光谱的分析可以看出,3组复合涂层中1 590 cm-1处的G 峰与1 352 cm-1处的G,D峰都非常突出,其中G 峰代表着氧化石墨烯的sp2晶体的形态,而D峰则反映了它的不规则的振荡行为。此外,50 V组峰值最大,其次为30 V组,而10 V组的峰值则最小,见图4。并且594,962,588 cm-1处均出现了PO43-的显著高位,而786 cm-1处则存在着Ti-O 的明显高位,这些高位的出现是由于羟基磷灰石中PO43-的存在所致。因此,发现这种涂料既包括了氧化石墨烯又包括羟基磷灰石晶体,而这些物质在这种情况下具有良好的互溶性。 2.1.4 复合涂层的水接触角测量 10,30,50 V组的静态接触角分别为(31.71±1.43)°,(24.38±1.21)°和(14.96±1.32)°,组间比较差异有显著性意义(P < 0.05),见图5。 综合以上实验结果,在50 V电压下制备的复合涂层物理性能最优,选择该复合涂层进行动物体内实验。"

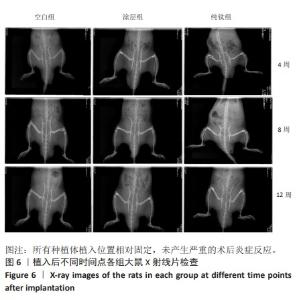
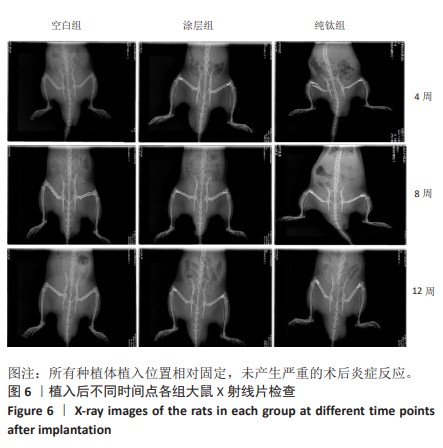
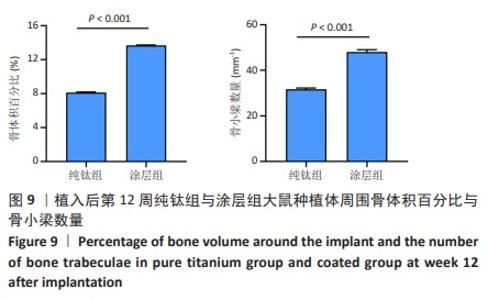
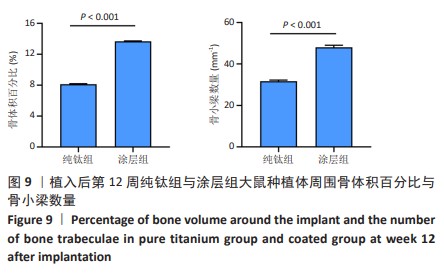
2.2.4 各组大鼠种植体Micro-CT扫描结果 因空白组未植入种植体,所以未进行Micro-CT扫描。纯钛组与涂层组大鼠植入体和周围骨组织的3D重建图如图8所示,图中灰白色代表植入体,黄色代表骨组织。植入后第4周,涂层组骨组织已基本覆盖种植体表面1/2左右,纯钛组植入体表面骨组织较涂层组明显减少。植入后第8周,相较于纯钛组,涂层组种植体表面覆盖的新骨明显更为致密,成骨量也相对更多。植入后第12周,纯钛组种植体表面还有少量区域未被骨组织覆盖,涂层组可见骨组织基本覆盖了种植体表面。植入后第12周,两组大鼠种植体周围骨组织骨体积百分比、骨小梁数量定量分析结果,见图9,涂层组大鼠种植体周围骨组织骨体积百分比、骨小梁数量均高于纯钛组(P < 0.001)。"

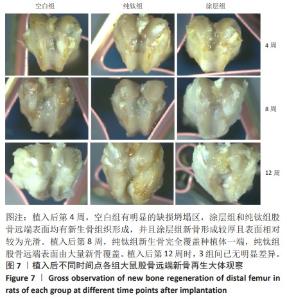
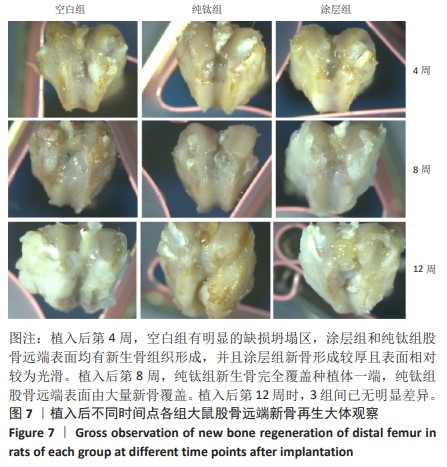
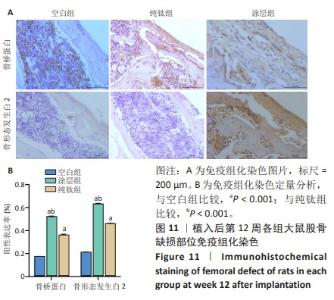
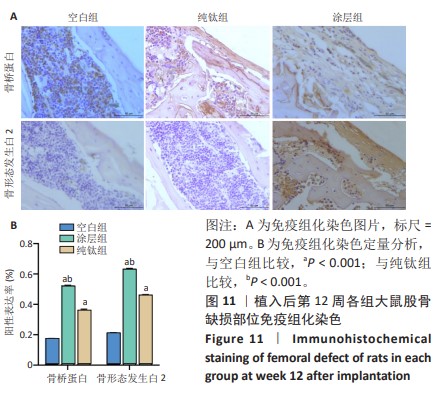
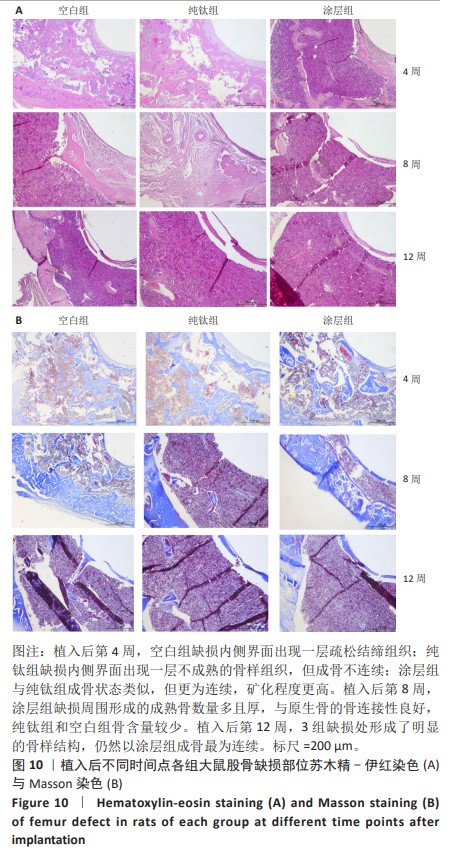
2.2.5 各组大鼠股骨缺损部位苏木精伊红染色与Masson染色 各组大鼠股骨缺损部位苏木精-伊红染色与Masson染色,见图10。 苏木精-伊红染色染色与Masson染色结果显示,植入后第4周,空白组缺损内侧界面出现一层疏松结缔组织;纯钛组缺损内侧界面出现一层不成熟的骨样组织,但成骨不连续;涂层组与纯钛组成骨状态类似,但更为连续,矿化程度更高。植入后第8周,涂层组缺损周围形成的成熟骨数量多且厚,与原生骨的骨连接性良好,而纯钛组和空白组骨含量较少,并且缺乏完整性。植入后第12周,3组缺损处形成了明显的骨样结构,仍然以涂层组成骨最为连续。 2.2.6 各组大鼠股骨缺损部位免疫组化染色 植入后第12周各组大鼠股骨缺损部位免疫组化染色,见图11所示。纯钛组大鼠骨缺损部位骨桥蛋白、骨形态发生蛋白2表达多于空白组,涂层组大鼠骨缺损部位骨桥蛋白、骨形态发生蛋白2表达多于纯钛组(P < 0.001)。 综上所述,上述实验结果显示羟基磷灰石-氧化石墨烯复合涂层在钛基质上的多层结构可以通过调节细胞黏附、上调相关基因的表达,同时有效释放Ca、P基质,达到改善植体表面骨形成的作用。"
| [1] 于婉琦,周延民,赵静辉.口腔种植体新材料的研究现状[J]国际口腔医学杂志,2019,46(4):488-496. [2] KONISHI T, HONDA M, NAGAYA M, et al. Injectable chelate-setting hydroxyapatite cement prepared by using chitosan solution: Fabrication, material properties, biocompatibility, and osteoconductivity. J Biomater Appl. 2017;31(10):1319-1327. [3] DE PAULA GA, SILVA GC, VILAÇA EL, et al. Biomechanical Behavior of Tooth-Implant Supported Prostheses With Different Implant Connections: A Nonlinear Finite Element Analysis. Implant Dent. 2018;27(3):294-302. [4] HSU KW, WEI PC, CHEN YL. Retrospective and clinical evaluation of afterma-rket CAD/CAM Titanium abutments supporting posterior splinted prosth-eses and single crowns. Int J Oral Maxillofac Implants. 2019;34(5):1161-1168. [5] O’HARE P, MEENAN BJ, BURKE GA, et al. Biological responses to hydroxyapatite surfaces deposited via a co-incident microblasting technique. Biomaterials. 2009;31(3):515-522. [6] 柏彬,肖玉周.骨组织工程的研究进展[J].解剖与临床,2010,15(4): 287-289. [7] ZAKARIA SM, SHARIF ZEIN SH, et al. Nanophase hydroxyapatite as a biomaterial in advanced hard tissue engineering: a review. Tissue Eng Part B Rev. 2013;19(5):431-41. [8] LEE C, WEI X, KYSAR JW, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008; 321(5887):385-388. [9] SHAMS SS, ZHANG R, ZHU J. Graphene synthesis: a review. Mater Sci-Poland. 2015; 33(3):566-578. [10] LIU LP, YANG XN, YE L, et al. Preparation and characte rizati on of a photocatalyti cantibacteri almaterial: graphene oxide/TiO2/bact erial cellulose nanocomposite. Carbohydr Polym. 2017;174:1078-1086. [11] LI Q, YONG CY, CAO WW, et al. Fabri cation of charge reve rsi ble graphe ne oxide -based nanocomposite with multiple antibacte rial modes and magnetic recycl ability. J Colloid Interface Sci. 2017;511:285-295. [12] JAIDEV LR, KUMAR S, CHATTERJEE K. Multi-biofunctional polymer graphene composite for bone tissue regeneration that elutes copper ions to impart angiogenic, osteogenic and bactericidal properties. Colloids Surf B Biointerfaces. 2017;159:293-302. [13] WANG Q, CHU Y, HE J, et al. A graded graphene oxide-hydroxyapatite/silkfibroin biomimetic scaffold for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;80:232-242. [14] SHI C, GAO J, WANG M, et al. Functional hydroxyapatite bioceramics with excellent osteoconductivity and stern-interface induced antibacterial ability. Biomater Sci. 2016;4(4):699-710. [15] STANGO S, KARTHICK D, SWAROOP S, et al. Development of hydroxyapatite coatings on laser textured 316 LSS and Ti-6Al-4V and its electrochemical beHAPvior in SBF solution for orthopedic applications. Ceram Int. 2017;44(3):3149-3160. [16] BARADARAN S, MOGHADDAM E, BASIRUN WJ, et al. Mechanical properties and biomedical applications of a nanotube hydroxyapatite reduced graphene oxide composite. Carbon. 2014;69:32-45. [17] GAO F, XU C, HU H, et al. Biomimetic synthesis and characterization of hydroxyapatite/graphene oxide hybrid coating on Mg alloy with enhanced corrosion resistance. Mater Lett. 2015;138:25-28. [18] WU C, XIA L, HAN P, et al. Graphene-oxide-modified β-tricalcium phosphate bioceramics stimulate in vitro and in vivo osteogenesis. Carbon. 2015;93:116-129. [19] PAPI M. Graphene-Based Materials: Biological and Biomedical Applications. Int J Mol Sci. 2021;22(2):672. [20] AZADIAN E, ARJMAND B, ARDESHIRYLAJIMI A, et al. Polyvinyl alcohol modified polyvinylidene fluoride-graphene oxide scaffold promotes osteogenic differentiation potential of human induced pluripotent stem cells. J Cell Biochem. 2020;121(5-6):3185-3196. [21] ARNOLD AM, HOLT BD, DANESHMANDI L, et al. Phosphate graphene as an intrinsically osteoinductive scaffold for stem cell-driven bone regeneration. Proc Natl Acad Sci USA. 2019;116(11):4855-4860. [22] JINLONG L, TONGXIANG L, CHEN W. Investigation of hydrogen evolution activity for the nickel, nickel-molybdenum nickel-graphite composite and nickel-reduced graphene oxide composite coatings. Appl Surf Sci. 2016;366:353-358. [23] 刘鹏,樊博,邹磊,等.钛基植入物抗菌/促成骨双功能表面改性策略研究进展[J].中国修复重建外科杂志,2023,37(10):1300-1313. [24] 李伶俐,李潇.钛基种植体表面涂层的研究进展[J].中国临床新医学,2020,13(5):527-532. [25] TARABALLI F, SUSHNITHA M, TSAO C, et al. Biomimetic tissue engineering:tuning the immune and inflammatory response to implantable biomaterials. Adv Healthc Mater. 2018;7(17):1800490. [26] VASCONCELOS DP, COSTA M, AMARAL IF, et al. Modulation of the inflammatory response to chitosan through M2 macrophage polarization using pro-resolution mediators. Biomaterials. 2015;37:116-123. [27] JÄMSEN E, KOURI VP, AINOLA M, et al. Correlations between macrophage polarizing cytokines, inflammatory mediators, osteoclast activity, and toll-like receptors in tissues around aseptically loosened hip implants. J Biomed Mater Res A. 2017;105(2):454-463. [28] CHENG H, XIONG W, FANG Z, et al. Strontium(Sr)and silver(Ag)loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta biomater. 2016;31:388-400. [29] KURAPATI R, MUKHERJEE SP, MARTÍN C, et al. Degradation of single-layer and few-layer graphene by neutrophil myeloperoxidase. Angew Chem Int Ed Engl. 2018;57(36):11722-11727. [30] LU YJ, WANG YH, SAHU RS, et al. Mechanism of Nanoformulated Graphene Oxide-Mediated Human Neutrophil Activation. ACS Appl Mater Interfaces. 2020;36:40141-40152. [31] LI K, SHEN Q, XIE Y, et al. Incorporation of cerium oxide into hydroxyapatite coating protects bone marrow stromal cells against H 2 O 2-induced inhibition of osteogenic differentiation. Biol Trace Elem Res. 2018;182:91-104. [32] HAN J, KIM YS, LIM MY, et al. Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair. Acs Nano. 2018;12(2):1959-1977. [33] FEITO MJ, DIEZ-OREJAS R, CICUÉNDEZ M, et al. Characterization of M1 and M2 polarization phenotypes in peritoneal macrophages after treatment with graphene oxide nanosheets. Colloids Surf B Biointerfaces. 2019;176:96-105. [34] CIANFEROTTI L, GOMES AR, FABBRI S, et al. The calcium-sensing receptor in bone metabolism:from bench to bedside and back. Osteop Int. 2015;26:2055-2071. [35] CHEN Z, WU C, GU W, et al. Osteogenic differentiation of bone marrow MSCs by β-tricalcium phosphate stimulating macrophages via BMP2signalling pathway. Biomaterials. 2014;35(5):1507-1518. [36] GONZÁLEZ-VÁZQUEZ A, PLANELL J A, ENGEL E. Extracellular calcium and CaSR drive osteoinduction in mesenchymal stromal cells. Acta Biomater. 2014;10(6):2824-2833. [37] BAEK SM, SHIN MH, MOON J, et al. Superior pre-osteoblast cell response of etched ultrafine-grained titanium with a controlled crystallographic orientation. Sci Rep. 2017;7(1):44213. [38] MADDEN LR, MORTISEN DJ, SUSSMAN EM, et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A. 2010;107(34):15211-15216. [39] AINSLIE KM, TAO SL, POPAT KC, et al. In vitro inflammatory response of nanostructured titania, silicon oxide, and polycaprolactone. J Biomed Mater Res A. 2009;91(3):647-655. |
| [1] | Yu Shuangqi, Ding Fan, Wan Song, Chen Wei, Zhang Xuejun, Chen Dong, Li Qiang, Lin Zuoli. Effects of polylactic acid-glycolic acid copolymer/lysine-grafted graphene oxide nanoparticle composite scaffolds on osteogenic differentiation of MC3T3 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 707-712. |
| [2] | Cheng Xinqi, Shao Longhui, Shen Huaqiao, Liu Hongwei. Osteogenic and antibacterial effects of titanium alloy modified with copper-strontium binary doped calcium silicate coating [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4639-4646. |
| [3] | Mawulanjiang · Abudurenmu, Zilalai · Julaiti, Baibujiafu · Yelisi, Gulizainu · Yibulayin, Nijiati · Tuerxun. Stress analysis of angled abutments of maxillary central incisor implant crown in different implant spacing [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3351-3359. |
| [4] | Guan Zhenju, Xie Yonglin, Xiang Shougang, Zhang Chengdong, Li Xiaolong, Li Xingping, Pu Chao, Zhang Bo, Luo Xuwei, Xiao Dongqin. Preparation of polyphenol-mediated copper ion coating on titanium surface and antibacterial and antioxidant properties [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 1997-2005. |
| [5] | Ma Lisha, He Huiyu, Wufanbieke·Baheti, Lyu Shangyi, Han Xiangzhen. effect of graphene oxide/hydroxyapatite composite coating on immune activity of RAW264.7 macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2023-2029. |
| [6] | Shi Qianhui, Wu Chao, Zhou Qian, Cheng Yuting, Li Fang, Huo Hua, Qi Yuhan, Huang Xiaolin, Wang Yong, Liao Jian. Prevention and treatment of implant periapical lesions [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5434-5440. |
| [7] | Chen Shaobo, Shuang Feng, Hu Wei, Li Hao, Shao Yinchu. Injectable bone graft for the repair of nonunion of midshaft clavicular fracture after internal fixation [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5473-5477. |
| [8] | Zhang Bin, Sun Lihua, Zhang Junhua, Liu Tongbin, Liu Yusan, Cui Caiyun, Li Jun. Short-term effect comparison of a modified socket shield technique and conventional flapless immediate implant and immediate restoration in maxillary aesthetic area [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5514-5519. |
| [9] | Zhang Suping, Sun Ling, Wan Dingming, Cao Weijie, Li Li, Liu Changfeng, Liu Yufeng, Wang Dao, Guo Rong, Jiang Zhongxing, Xie Xinsheng. Effectiveness of unrelated peripheral blood stem cell transplantation in the treatment of severe aplastic anemia [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(31): 4994-5001. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||