Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (8): 1676-1683.doi: 10.12307/2025.335
Previous Articles Next Articles
Mitophagy regulates bone metabolism
Zhu Hanmin1, Wang Song2, Xiao Wenlin3, Zhang Wenjing2, Zhou Xi2, He Ye2, Li Wei1, 2
- 1Medical College, Shaoxing University, Shaoxing 312099, Zhejiang Province, China; 2Medical College, Hubei University of Arts and Science, Xiangyang 441053, Hubei Province, China; 3Center for Disease Control and Prevention of Hainan, Haikou 570203, Hainan Province, China
-
Received:2024-02-04Accepted:2024-05-09Online:2025-03-18Published:2024-07-06 -
Contact:Li Wei, MD, Associate professor, Master’s supervisor, Medical College, Shaoxing University, Shaoxing 312099, Zhejiang Province, China; Medical College, Hubei University of Arts and Science, Xiangyang 441053, Hubei Province, China -
About author:Zhu Hanmin, Master, Laboratory technician, Medical College, Shaoxing University, Shaoxing 312099, Zhejiang Province, China -
Supported by:the National Natural Science Foundation of China (Youth Program), No. 82205234 (to LW); Key Project of Xiangyang Science and Technology Bureau, No. 2022YL07A (to LW); Clinical Medicine Special Fund Project of Zhejiang Provincial Medical Association, No. 2023ZYC-A182 (to LW); Hubei Provincial Natural Science Foundation, No. 2024AFD049 (to ZX)
CLC Number:
Cite this article
Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
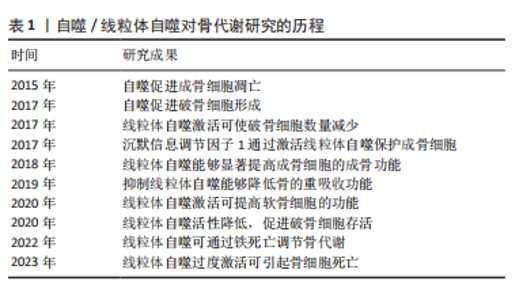
2.1 自噬/线粒体自噬对骨代谢研究的历程 自噬概念被提出以来,大量的基础和临床研究证明,自噬和线粒体自噬在骨代谢的调节过程中扮演着重要角色,非生理状态下的线粒体自噬会打破骨代谢的平衡,导致骨代谢紊乱[6-7],对成骨细胞、破骨细胞、骨细胞、软骨细胞、骨髓间充质干细胞等均可产生影响[8],如表1所示。自噬和线粒体自噬可通过多种信号通路调节骨代谢相关细胞的增殖、分化以及功能,主要包括PINK1/帕金森相关蛋白、沉默信息调节因子1(silence information regulator 1,SIRT1)、MAPK8/FOXO3a、自噬相关因子Beclin-1/BECN1、p62/自噬溶酶体底物蛋白-泛素结合蛋白核自噬受体及哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)通路。"
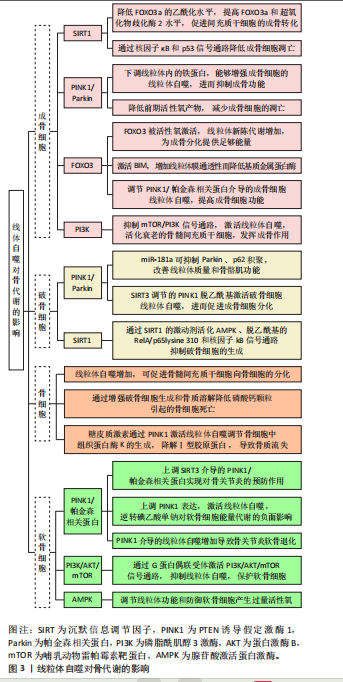
2.2 线粒体自噬对成骨细胞的影响 2.2.1 线粒体自噬途径SIRT1 SIRT是烟酰胺腺嘌呤二核苷酸依赖性脱乙酰酶的高度保守蛋白家族,参与调节细胞和代谢功能,包括抗逆性、基因组稳定性、肿瘤发生和能量代谢[9]。SIRT1是酵母沉默信息调节因子2的同源物[10],可通过多种途径调控线粒体自噬,维持线粒体正常的生理功能[11]。由SIRT1介导的过氧化物酶体增殖活化受体γ辅助活化因子1α(the transcriptional co-activator peroxisome proliferator-activated receptor gamma coactivator 1 alpha,PGC-1α)去乙酰化,在线粒体代谢调控和线粒体生成过程起关键作用[12]。诸多研究证实,SIRT1表达增加可以显著激活线粒体自噬[13-14]。 SIRT1是骨代谢的重要调节因子。COHEN-KFIR等[15]发现敲除SIRT1基因会导致雌性小鼠骨质大量流失和间充质干细胞的成骨转化减少、成脂生成增加。SUN等[16]证明在间充质干细胞中过表达SIRT1可抑制FOXO3a的乙酰化并增加FOXO3a和超氧化物歧化酶2的表达,进而促进间充质干细胞的成骨转化和减缓衰老。同时,磨损颗粒可降低动物模型中成骨细胞和骨质溶解动物模型中SIRT1的表达,反之,增加成骨细胞中SIRT1的表达可以显著降低磨损颗粒诱导的炎性细胞因子表达和核因子κB/p53信号介导的成骨细胞凋亡 [17]。 2.2.2 线粒体自噬途径PINK1/帕金森相关蛋白 PINK1/帕金森相关蛋白通路是调控线粒体自噬的经典通路[18]。PINK1是一种丝氨酸/苏氨酸激酶,是帕金森相关蛋白介导线粒体自噬过程中必需的蛋白[19-20]。PINK1拥有1个N端线粒体靶向序列、1个α螺旋跨膜片段和1个丝氨酸/苏氨酸激酶结构域。帕金森相关蛋白是一种E3-泛素连接酶,被PINK1选择性地募集到具有低膜电位且功能失调线粒体上[21]。在应激及病理条件下,PINK1感知线粒体损伤并聚集在线粒体外膜上,促进帕金森相关蛋白转位到线粒体外膜,PINK1在Ser65处磷酸化帕金森相关蛋白和Ub,磷酸化的帕金森相关蛋白将泛素与各种线粒体外膜蛋白偶联,继而诱导p62聚集并与LC3特异性结合,从而介导线粒体自噬 [22]。 PINK1/帕金森相关蛋白信号通路可通过多种途径参与调节成骨代谢。正常情况下,线粒体中的活性氧储存在线粒体内,但当线粒体功能受损时这些活性氧将会被释放到细胞质中,进一步加剧细胞的氧化应激,导致细胞凋亡[23]。研究表明,雷帕霉素可以通过PINK1/帕金森相关蛋白信号通路调控线粒体自噬,降低前期活性氧产物,减少成骨细胞的凋亡[24]。PINK1/帕金森相关蛋白诱导的线粒体自噬也参与调节间充质干细胞的成骨分化[25]。另外,铁死亡作为近年最新发现的细胞死亡方式可以影响骨的重塑,包括骨的重吸收和形成[26]。尽管目前已有研究表明,下调线粒体内的铁蛋白能够通过PINK1/帕金森相关蛋白途径增加线粒体自噬,进而促进成骨细胞内的铁死亡,抑制成骨功能[27],但是线粒体自噬调控铁死亡的具体机制尚未阐明,其对骨代谢的影响机制也不是很清楚,故靶向调节铁死亡有望成为通过线粒体自噬途径有效改善骨代谢的潜在靶点。 2.2.3 线粒体自噬途径FOXO3 FOXO是一个转录因子家族,在细胞处于氧化应激状态时维持细胞稳态起着至关重要的作用。FOXO家族包括4个成员,分别是FOXO1、FOXO3、FOXO4和FOXO6。GOMEZ-PUERTO等[28]研究发现,在人间充质干细胞的成骨分化过程中FOXO3被活性氧激活,线粒体新陈代谢增加以提供成骨分化过程所需的足够能量。以上过程主要依赖于MAPK8诱导FOXO3的Ser294位点磷酸化,而FOXO3的活化在通过激活自噬控制活性氧水平中具有重要作用。FoxO3a的激活可能通过激活BH3结构域唯一蛋白Bim增加线粒体膜通透性,从而降低线粒体膜电位[29]。ZHAO等[30]的研究证明,高选择性镁转运体可通过调控细胞内Mg2+水平增加hFOB1.19细胞的线粒体膜电位,减弱PINK1/帕金森相关蛋白介导的线粒体自噬,并且高糖处理过表达高选择性镁转运体的hFOB1.19细胞可显著改善PGC-1α的减少和FoxO3a的失活,故提出高选择性镁转运体通过PGC-1α/FoxO3a/线粒体膜电位信号通路调节PINK1/帕金森相关蛋白介导的成骨细胞线粒体自噬,提高成骨细胞的功能。 2.2.4 线粒体自噬途径PI3K 磷脂酰肌醇3激酶(phosphatidyqinositol-3 kinase,PI3K)属于脂质激酶家族,参与真核生物细胞膜的形成,调节细胞信号转导、能量代谢、细胞周期等细胞内的过程[31]。PI3K/蛋白激酶B(protein kinase B,Akt)信号通路在调控细胞凋亡和线粒体自噬方面发挥重要作用,Akt可通过磷酸化阻止促凋亡蛋白和线粒体自噬相关蛋白插入线粒体外膜,减少线粒体膜电位的损坏,同时该通路也会减少线粒体活性氧的产生,抑制凋亡和自噬[32-35]。 在囊泡成核期间,通过PI3K介导的自噬体现出了双重功能,一方面是抑制自噬,另一方面是激活自噬[36-37],并且PI3K也参与调节线粒体自噬[38]。LIU 等[39]的研究发现,富亮氨酸重复序列蛋白17被敲减后能够通过抑制mTOR/PI3K信号通路激活线粒体自噬,进而减轻线粒体功能紊乱,重新活化已经衰老的骨髓间充质干细胞并发挥成骨作用,最终缓解骨质疏松。此外,有学者提出PI3K通路是把双刃剑,PI3K对线粒体自噬的抑制或促进取决于细胞处于的微环境[40]。 2.3 线粒体自噬对破骨细胞的影响 线粒体自噬在破骨细胞骨吸收及存活过程中发挥着重要作用。破骨细胞骨吸收及降解过程中会释放溶酶体,激活骨吸收蛋白的表达进而降解骨质,并诱导自噬标志蛋白LC3定位于破骨细胞褶皱边缘,促进破骨细胞发生自噬性死亡[41]。线粒体自噬激活后,一方面破骨细胞骨吸收活性增强,另一方面影响破骨细胞的存活,提示干预破骨细胞线粒体自噬可能是抑制破骨细胞骨吸收活性及存活的潜在机制。 骨质疏松大鼠骨量丢失(骨体积分数、骨密度降低)增加,破骨细胞凋亡率降低、存活率升高,而破骨细胞中线粒体自噬形成的溶酶体及自噬体增多,PINK1/帕金森相关蛋白自噬信号通路激活,证实骨质疏松大鼠破骨细胞存活数量增加,可能与线粒体自噬激活有关[42]。另有动物实验研究表明,敲除SIRT3基因或者使用SIRT3抑制剂能够部分抑制破骨细胞的形成和骨吸收的功能,证实SIRT3调节的PINK1脱乙酰基可以通过激活破骨细胞线粒体自噬,进而促进成骨细胞分化[43]。除了SIRT3,SIRT1在破骨细胞生成、分化及发挥重吸收功能中也具有重要作用,如SIRT1的激动剂SRT2183和SRT3025能够通过活化腺苷酸激活蛋白激酶(adenosine monophosphate activated protein kinase,AMPK)、脱乙酰基的 RelA/p65 lysine 310和核因子κB信号通路抑制破骨细胞的生成。 近年来的研究发现,miRNA与骨质疏松中破骨细胞活性增强关系密切[25],其中miR-181a可靶向上调死亡受体相关蛋白的表达,促进破骨细胞的增殖及分化[44],从而影响骨质疏松进程。miR-181a是一种线粒体自噬抑制因子,GOLJANEK-WHYSALL等[45]的研究发现,骨骼功能异常及肌肉萎缩与miR-181a水平降低以及线粒体自噬相关蛋白、p62、SQSTM1积聚有关,上调miR-181a可抑制帕金森相关蛋白、p62积聚,改善线粒体质量和骨骼肌功能。miR-181a与帕金森相关蛋白之间存在靶向负调控关系,上调骨质疏松大鼠破骨细胞中miR-181a的表达后,帕金森相关蛋白介导的自噬活性降低,破骨细胞细胞存活率升高、凋亡率降低,推测抑制骨质疏松大鼠破骨细胞自噬可促进破骨细胞存活,这与SHARMA等[46]研究的抑制线粒体自噬可减轻细胞过度活化产生的应激性损伤减缓细胞自噬性死亡的观点相一致。反之下调miR-181a的表达,帕金森相关蛋白介导的自噬进一步被激活,破骨细胞存活率降低,提示下调miR-181a的表达可能会促进破骨细胞发生自噬性死亡,这与既往研究中下调miR-181a表达可抑制破骨细胞活性以及自噬过度激活导致细胞因能量溃乏而死亡的结论相一致[47]。另外,外源性敲除破骨细胞中的帕金森相关蛋白基因,可逆转下调miR-181a发挥的促进自噬及促凋亡作用。 2.4 线粒体自噬对骨细胞的影响 骨髓间充质干细胞是成体干细胞,可以增殖和分化为成熟的基质细胞,如骨细胞、脂肪细胞等[48]。骨髓间充质干细胞与骨质疏松等年龄相关的代谢性密切相关,当前研究已经证明,骨髓间充质干细胞具有潜在分化为成骨细胞的作用以及通过旁分泌作用促进成骨和生成血管,故其与骨质疏松的发生密切相关[49]。线粒体自噬在促进骨髓间充质干细胞线粒体分裂增强干性中发挥关键作用,促进线粒体分裂或抑制线粒体自噬均可抑制骨髓间充质干细胞的活性[50]。另有研究表明,线粒体自噬可以通过增强破骨细胞生成和骨质溶解调节磷酸钙颗粒引起的骨细胞死亡 [51]。糖皮质激素也可通过PINK1激活线粒体自噬调节骨细胞中组织蛋白酶K的产生,进而降解Ⅰ型胶原蛋白,导致骨质流失[52]。 2.5 线粒体自噬对软骨细胞的影响 软骨细胞是由骨祖细胞分化而来,位于软骨陷窝内,具有合成和分泌基质与纤维的功能,对关节的承重和缓冲功能具有重要意义。软骨细胞的线粒体氧化磷酸化是其代谢所需ATP的主要来源[53]。研究发现,线粒体形态和功能的改变与骨关节炎的发病机制密切相关[54]。因此,线粒体稳态对软骨细胞的存活和功能至关重要。研究发现亚精胺可有效减弱自噬流,并可通过增加线粒体自噬标志物的表达激活线粒体自噬,进而对抗氧化应激,从而起到保护软骨的作用[55]。研究表明,多条线粒体自噬相关的信号通路与骨关节炎的发病机制相关,如 AMPK[56]、BNIP3[57]、PINK1和mTOR[58-59]。 线粒体自噬是一种选择性自噬过程,涉及清除受损线粒体和维持线粒体稳态,促进骨关节炎相关基因表达,二甲双胍通过上调SIRT3介导的PINK1/帕金森相关蛋白依赖性线粒体自噬,实现对骨关节炎的预防作用[60]。在碘乙酸单钠处理的软骨细胞骨关节炎模型中,锌可逆转碘乙酸单钠对软骨细胞能量代谢的负面影响,并最终上调PINK1表达,激活线粒体自噬 [61]。反之,在骨关节炎大鼠模型和人类原发性骨关节炎患者的软骨细胞中,PINK1介导的线粒体自噬增加导致骨关节炎软骨退化[58]。植物同源域脂蛋白23是一种通过促进E3连接酶降解的新型自噬抑制剂,在人骨关节炎软骨和滑膜中过表达[62],敲除植物同源域脂蛋白23可增加线粒体自噬和Ⅱ型胶原蛋白表达并减少骨关节炎相关蛋白表达,防止软骨细胞凋亡,故植物同源域脂蛋白23极可能成为骨关节炎的有效治疗靶点[63]。类固醇激素17β-雌二醇通过G蛋白偶联雌激素受体激活PI3K/AKT/mTOR信号通路来抑制线粒体自噬,从而对骨关节炎中的软骨细胞发挥保护作用[64]。此外,BLANCO等 [65]也提出,AMPK-SIRT-帕金森相关蛋白信号通路在调节线粒体功能和防御骨关节炎软骨细胞产生过量活性氧时具有关键作用。线粒体自噬对骨代谢的影响见图3。 2.6 线粒体自噬应用于骨代谢领域的前景展望 绝经后骨质疏松和老年性骨质疏松症的主要诱因是激素水平降低导致骨代谢紊乱。17β-雌二醇缺乏是绝经后骨质疏松的主要原因之一,17β-雌二醇通过G蛋白偶联雌激素受体30/ERK1/2信号通路诱导线粒体自噬,保护成骨细胞。然而,当用G15(一种选择性G蛋白偶联雌激素受体30拮抗剂[66])预处理细胞时,雌激素的保护作用被阻断。除了G蛋白偶联雌激素受体30/ERK1/2信号通路外,自噬还可通过Wnt、骨形态发生蛋白/Smad、核因子κB受体激活因子配体/ 核因子κB的受体激活因子、肿瘤坏死因子α及mTOR等信号通路控制绝经后骨质疏松的发展[67-68]。此外,由于氧化应激作为骨质疏松中成骨细胞骨形成受损的关键因素,因此可通过葡萄糖调节蛋白78和蛋白激酶样内质网激酶来缓解早期的成骨细胞自噬[69]。SIRT1是线粒体自噬的关键调控因子,临床上靶向调控SIRT1的候选药物在治疗骨质疏松症和其他骨代谢疾病方面具有良好的效果[70]。 此外,中药通过线粒体自噬途径调控骨质疏松的研究和应用也比较广泛。从中药益母草中提取的天然化合物——益母草碱因强大的抗氧化作用而受到广泛关注[71]。ZHAO等[72]的研究表明,益母草可通过抑制PI3K/Akt/mTOR信号通路激活线粒体自噬,减少骨髓间充质干细胞中活性氧的产生,维持线粒体功能和质量,从而保护骨髓间充质干细胞的增殖和分化作用。这些研究为中药成分通过线粒体自噬促进骨髓间充质干细胞的成骨分化提供了证据。成骨细胞功能障碍是导致骨丢失的主要原因之一,探究中药成分是否可通过线粒体自噬途径调节成骨细胞功能也是治疗骨代谢疾病的重要途径[73]。YANG等[74]报道中药成分白藜芦醇通过上调SIRT1的表达激活PI3K/Akt/mTOR信号通路,增强骨质疏松大鼠成骨细胞线粒体"

自噬,显著增加骨质疏松大鼠的骨量,降低血清中碱性磷酸酶和骨钙素水平。 骨肉瘤是发生于儿童和青壮年的常见恶性骨肿瘤,具有易复发、侵袭性强、早期转移的特点[75-76],常规手术治疗可能会出现肢体残疾等并发症,导致预后不良。近年来,新型辅助化疗已成为治疗骨肉瘤的常见的策略,但也存在耐药和不良反应大等缺点[77],因此迫切需要找到新的有效治疗靶点,进而发现更有效的骨肉瘤治疗方法。自噬为细胞的自我保护机制,失调的自噬参与多种病理过程,也包括癌症[78]。由于线粒体的功能及质量在肿瘤细胞中经常发生改变(Warbung效应),癌症代谢高度依赖于糖酵解[79]。线粒体依赖性凋亡通过线粒体自噬选择性降解来维持细胞的平衡[80]。线粒体自噬在抗癌药物中具有细胞保护作用[81-82]。前期研究发现,抗肿瘤药物去甲斑蝥素通过线粒体自噬抑制人骨肉瘤细胞增殖并促进其凋亡[83],抗炎植物药的有效化学成分小白菊内酯也可通过线粒体自噬诱导骨肉瘤细胞死亡[84]。以上结果提示线粒体自噬参与骨肉瘤细胞凋亡的调控。 另有学者发现,在化疗过程中骨肉瘤细胞死亡可能与PINK1/帕金森相关蛋白介导的线粒体自噬有关[84]。VIANELLO等[85]的研究发现,在体外顺铂耐药模型和对铂类化疗耐药的癌症患者样本中,线粒体自噬因子BNIP3表达水平较高,线粒体自噬增强;进一步的研究发现,降低BNIP3表达可抑制线粒体自噬,进而使耐药细胞对顺铂重新增敏,提示BNIP3可能是克服骨肉瘤治疗时耐药现象的潜在靶点,这些研究为BNIP3介导的线粒体自噬在不同类型癌症中的应用提供了充分证据。ZnO纳米颗粒是一种潜在的治疗癌症纳米药物,HE等[86-87]的研究发现,ZnO纳米颗粒诱导的骨肉瘤细胞凋亡是通过缺氧诱导因子1-BNIP3-LC3b介导的线粒体自噬途径实现的,ZnO纳米颗粒通过内吞途径进入人骨肉瘤细胞,在被溶酶体捕获之前破坏线粒体,从而启动线粒体自噬-Zn2+-活性氧-线粒体轴介导的骨肉瘤细胞凋亡。"

| [1] 刘垒,冯杜,朱玉山,等.线粒体自噬的研究进展[J].中国细胞生物学学报,2012,34(10):959-966. [2] 郭兴,臧珊珊,张怀东,等.病毒感染与宿主细胞线粒体动力学及线粒体自噬[J].生命科学,2021,33(11):1332-1338. [3] 程婧,魏林,李苗.线粒体动力学及线粒体自噬调控机制的研究进展[J].生理学报,2020,72(4):475-487. [4] TWIG G, ELORZA A, MOLINA AJA, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433-446. [5] KHAN M, SYED GH, KIM SJ, et al. Mitochondrial dynamics and viralinfections: a close nexus. Biochim Biophys Acta. 2015;1853:2822-2833. [6] WANG Z, LIU N, LIU K, et al. Autophagy mediated CoCrMo particle-induced peri-implant osteolysis by promoting osteoblast apoptosis.Autophagy. 2015;11:2358-23569. [7] WANG Z, DENG Z, GAN J, et al. TiAl6V4 particles promote osteoclast formation via autophagy-mediated downregulation of interferon-beta in osteocytes. Acta Biomater. 2017;48:489-498. [8] WANG S, DENG Z, MA Y, et al. The Role of Autophagy and Mitophagy in Bone Metabolic Disorders. Int J Biol Sci. 2020;16(14):2675-2691. [9] FINKEL T, DENG CX, MOSTOSLAVSKY R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587-591. [10] RINE J, STRATHERN JN, HICKS JB, et al. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics. 1979;93:877-901. [11] YOSHII SR, MIZUSHIMA N. Autophagy machinery in the context of mammalian mitophagy. Biochim Biophys Acta. 2015;1853:2797-2801. [12] TANG BL. Sirt1 and the Mitochondria. Mol Cells. 2016;39:87-95. [13] JANG SY, KANG HT, HWANG ES. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biol Chem. 2012;287:19304-19314. [14] DI SANTE G, PESTELL TG, CASIMIRO MC, et al. Loss of Sirt1 promotes prostatic intraepithelial neoplasia, reduces mitophagy, and delays PARK2 translocation to mitochondria. Am J Pathol. 2015;185:266-279.
[15] COHEN-KFIR E, ARTSI H, LEVIN A, et al. Sirt1 Is a Regulator of Bone Mass and a Repressor of Sost Encoding for Sclerostin, a Bone Formation Inhibitor. Endocrinology. 2011;152:4514-4524. [16] SUN W, QIAO W, ZHOU B, et al. Overexpression of Sirt1 in mesenchymal stem cells protects against bone loss in mice by FOXO3a deacetylation and oxidative stress inhibition. Metabolism. 2018;88:61-71. [17] DENG Z, WANG Z, JIN J, et al. SIRT1 protects osteoblasts against particle-induced inflammatory responses and apoptosis in aseptic prosthesis loosening. Acta Biomater. 2017;49:541-554. [18] PLOUMI C, DASKALAKI I, TAVERNARAKIS N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 2017;284:183-195. [19] NARENDRA DP, JIN SM, TANAKA A, et al. PINK1 Is Selectively Stabilized on Impaired Mitochondria to Activate Parkin. Plos Biol. 2010;8(1): e1000298. [20] KAWAJIRI S, SAIKI S, SATO S, et al. PINK1 is recruited to mitochondria with parkin and associates with LC3 in mitophagy. Febs Letters. 2010; 584:1073-1079. [21] NARENDRA D, TANAKA A, SUEN DF, et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795-803. [22] ZHANG Z, CHEN Z, LIU R, et al. Bcl-2 proteins regulate mitophagy in lipopolysaccharide-induced acute lung injury via PINK1/Parkin signaling pathway. Oxid Med Cell Longev. 2020;6579696. doi: 10.1155/2020/6579696. [23] ZOROV DB, JUHASZOVA M, SOLLOTT SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909-950. [24] LI W, JIANG WS, SU YR, et al. PINK1/Parkin-mediated mitophagy inhibits osteoblast apoptosis induced by advanced oxidation protein products. Cell Death Dis. 2023;14(2):88. [25] 陈施羊,周爱国,闫文龙,等.高迁移率族蛋白1通过PINK1/Parkin介导的线粒体自噬促进骨髓干细胞的趋化及成骨分化[J].骨科, 2023,14(5):445-452. [26] GAO Z, CHEN Z, XIONG Z, et al. Ferroptosis—A new target of osteoporosis. Exp Gerontol. 2022;165:111836. [27] WANG X, MA H, SUN J, et al. Mitochondrial Ferritin Deficiency Promotes Osteoblastic Ferroptosis Via Mitophagy in Type 2 Diabetic Osteoporosis. Biol.Trace Elem Res. 2022;200:298-307. [28] GOMEZ-PUERTO MC, VERHAGEN LP, BRAAT AK, et al. Activation of autophagy by FOXO3 regulates redox homeostasis during osteogenic differentiation. Autophagy. 2016;12:1804-1816. [29] SHUKLA S, RIZVI F, RAISUDDIN S, et al. FoxO proteins’ nuclear retention and BH3-only protein Bim induction evoke mitochondrial dysfunction-mediated apoptosis in berberine-treated HepG2 cells. Free Radic Biol Med. 2014;76:185-199. [30] ZHAO W, ZHANG W, MA H, et al. NIPA2 regulates osteoblast function by modulating mitophagy in type 2 diabetes osteoporosis. Sci Rep. 2020;10(1):3078. [31] LIU B, CAO Y, WANG D, et al. Zhen-Wu-Tang Induced Mitophagy to Protect Mitochondrial Function in Chronic Glomerulonephritis via PI3K/AKT/mTOR and AMPK Pathways. Front Pharmacol. 2021;12: 777670. [32] SCHAUER A, BARTHEL P, ADAMS V, et al. Pharmacological Pre- and Postconditioning With Levosimendan Protect H9c2 Cardiomyoblasts From Anoxia/Reoxygenation-induced Cell Death via PI3K/Akt Signaling. J Cardiovasc Pharmacol. 2021;77(3):378-385. [33] BAGHERY SAGHCHY KHORASANI A, POURBAGHERI-SIGAROODI A, PIRSALEHI A, et al. The PI3K/Akt/mTOR signaling pathway in gastric cancer; from oncogenic variations to the possibilities for pharmacologic interventions. Eur J Pharmacol. 2021;898:173983. [34] MEERAN MFN, AZIMULLAH S, ADEGHATE E, et al. Nootkatone attenuates myocardial oxidative damage, inflammation, and apoptosis in isoproterenol-induced myocardial infarction in rats. Phytomedicine. 2020;84:153405. [35] KIM SY, HWANGBO H, KIM MY, et al. Coptisine induces autophagic cell death through down-regulation of PI3K/Akt/mTOR signaling pathway and up-regulation of ROS-mediated mitochondrial dysfunction in hepatocellular carcinoma Hep3B cells. Arch Biochem Biophys. 2021; 697:108688. [36] AHSAN A, LIU M, ZHENG Y, et al. Natural compounds modulate the autophagy with potential implication of stroke. Acta Pharm Sin B. 2021;11:1708-1720. [37] ABID R, GHAZANFAR S, FARID A, et al. Pharmacological Properties of 40, 5, 7-Trihydroxyflavone (Apigenin) and Its Impact on Cell Signaling Pathways. Molecules. 2022;27:4304. [38] MAITI P, SCOTT J, SENGUPTA D, et al. Curcumin and Solid Lipid Curcumin Particles Induce Autophagy, but Inhibit Mitophagy and the PI3K-Akt/mTOR Pathway in Cultured Glioblastoma Cells. Int J Mol Sci. 2019;20:399. [39] LIU F, YUAN Y, BAI L, et al. LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss. Redox Biol. 2021;43:101963. [40] YIN Y, DANG W, ZHOU X, et al. PI3K-Akt-mTOR axis sustains rotavirus infection via the 4E-BP1 mediated autophagy pathway and represents an antiviral target. Virulence. 2018;9:83-98. [41] ZHENG Z, ZHANG X, HUANG B, et al. Site-1 protease controls osteoclastogenesis by mediating LC3 transcription. Cell Death Differ. 2021;28(6):2001-2018. [42] LI J, YANG S, LI X, et al. Role of endoplasmic reticulum stress in disuse osteoporosis. Bone. 2017;97:2-14. [43] LING W, KRAGER K, RICHARDSON KK, et al. Mitochondrial Sirt3 contributes to the bone loss caused by aging or estrogen deficiency. JCI Insight. 2021;6:e146728. [44] SHAO B, FU X, YU Y, et al. Regulatory effects of miRNA-181a on FasL expression in bone marrow mesenchymal stem cells and its effect on CD4+ T lymphocyte apoptosis in estrogen deficiency-induced osteoporosis. Mol Med Rep. 2018;18(1):920-930. [45] GOLJANEK-WHYSALL K, SORIANO-ARROQUIA A, MCCORMICK R, et al. miR-181a regulates p62/SQSTM1, parkin, and protein DJ-1 promoting mitochondrial dynamics in skeletal muscle aging. Aging Cell. 2020;19(4):e13140. [46] SHARMA LK, TIWARI M, RAI NK, et al. Mitophagy activation repairs Lebers here ditary optic neuropathy-associated mitochondrial dysfunction and improves cell survival. Hum Mol Genet. 2019;28(3): 422-433. [47] 高敬,邵秉一.miR-181a调控骨髓间充质干细胞中OPG水平及对破骨细胞活性的影响[J].中国细胞生物学学报,2017,39(1):44-51. [48] ZHANG Y, CHEN CY, LIU YW, et al. Neuronal Induction of Bone-Fat Imbalance through Osteocyte Neuropeptide Y. Adv Sci (Weinh). 2021; 8(24):e2100808. [49] KIERNAN J, HU S, GRYNPAS MD, et al. Systemic Mesenchymal Stromal Cell Transplantation Prevents Functional Bone Loss in a Mouse Model of Age-Related Osteoporosis. Stem Cells Transl Med. 2016;5:683-693. [50] FENG X, YIN W, WANG J, et al. Mitophagy promotes the stemness of bone marrow-derived mesenchymal stem cells. Exp Biol Med (Maywood). 2021;246(1):97-105. [51] YANG P, XU B, ZHU R, et al. ROS-mediated mitophagy and necroptosis regulate osteocytes death caused by TCP particles in MLO-Y4 cells. Toxicology. 2023;496:153627.
[52] YUAN J, GAO YS, LIU DL, et al. PINK1-mediated mitophagy contributes to glucocorticoid-induced cathepsin K production in osteocytes. J Orthop Translat. 2022;38:229-240.
[53] SU XJ, ZHENG C, HU QQ, et al. Bimetallic cooperative effect on O-O bond formation: Copper polypyridyl complexes as water oxidation catalyst. Dalton Trans. 2018;47:8670-8675. [54] HE Y, WU Z, XU L, et al. The role of SIRT3-mediated mitochondrial homeostasis in osteoarthritis. Cell Mol Life Sci. 2020;77:3729-3743. [55] D’ADAMO S, CETRULLO S, GUIDOTTI S, et al. Spermidine rescues the deregulated autophagic response to oxidative stress of osteoarthritic chondrocytes. Free Radic Biol Med. 2020;153:159-172. [56] MEI R, LOU P, YOU G, et al. 17-Estradiol Induces Mitophagy Upregulation to Protect Chondrocytes via the SIRT1-Mediated AMPK/mTOR Signaling Pathway. Front Endocrinol. 2020;11:615250. [57] KUWAHARA M, AKASAKI Y, KURAKAZU I, et al. C10orf10/DEPP activates mitochondrial autophagy and maintains chondrocyte viability in the pathogenesis of osteoarthritis. FASEB J. 2022;36:e22145. [58] SHIN HJ, PARK H, SHIN N, et al. Pink1-Mediated Chondrocytic Mitophagy Contributes to Cartilage Degeneration in Osteoarthritis. J Clin Med. 2019;8:1849. [59] ZHANG J, HAO X, CHI R, et al. Moderate mechanical stress suppresses the IL-1 -induced chondrocyte apoptosis by regulating mitochondrial dynamics. J Cell Physiol. 2021;236:7504-7515. [60] WANG C, YANG Y, ZHANG Y, et al. Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci Trends. 2019;12:605-612. [61] HUANG LW, HUANG TC, HU YC, et al. Zinc protects chondrocytes from monosodium iodoacetate-induced damage by enhancing ATP and mitophagy. Biochem Biophys Res Commun. 2020;521:50-56. [62] LI X, YANG X, MAIMAITIJUMA T, et al. Plant homeodomain finger protein 23 inhibits autophagy and promotes apoptosis of chondrocytes in osteoarthritis. Chin Med J (Engl). 2019;132:2581-2587. [63] MAIMAITIJUMA T, YU JH, REN YL, et al. PHF23 negatively regulates the autophagy of chondrocytes in osteoarthritis. Life Sci. 2020;253:117750. [64] FAN DX, YANG XH, LI YN, et al. 17β-Estradiol on the Expression of G-Protein Coupled Estrogen Receptor (GPER/GPR30) Mitophagy, and the PI3K/Akt Signaling Pathway in ATDC5 Chondrocytes In Vitro. Med Sci Monit. 2018;24:1936-1947. [65] BLANCO FJ, REGOPEREZ I. Mitochondria and mitophagy: biosensors for cartilage degradation and osteoarthritis. Osteoarthritis Cartilage. 2018;26:989-991. [66] SUN X, YANG X, ZHAO Y, et al. Effects of 17beta-Estradiol on Mitophagy in the Murine MC3T3-E1 Osteoblast Cell Line is Mediated via G Protein-Coupled Estrogen Receptor and the ERK1/2 Signaling Pathway. Med Sci Monit. 2018;24:903-911. [67] SHEN G, REN H, QIU T, et al. Implications of the Interaction Between miRNAs and Autophagy in Osteoporosis. Calcif Tissue Int. 2016;99:1-12. [68] QI M, ZHANG L, MA Y, et al. Autophagy Maintains the Function of Bone Marrow Mesenchymal Stem Cells to Prevent Estrogen Deficiency-Induced Osteoporosis. Theranostics. 2017;7:4498-4516. [69] YANG YH, LI B, ZHENG XF, et al. Oxidative damage to osteoblasts can be alleviated by early autophagy through the endoplasmic reticulum stress pathway-implications for the treatment of osteoporosis. Free Radic Biol Med. 2014;77:10-20. [70] ZAINABADI K. Drugs targeting SIRT1, a new generation of therapeutics for osteoporosis and other bone related disorders? Pharmacol Res. 2019;143:97-105. [71] CHEN C, ZHU Z, HU N, et al. Leonurine Hydrochloride Suppresses Inflammatory Responses and Ameliorates Cartilage Degradation in Osteoarthritis via NF-kB Signaling Pathway. Inflammation. 2020;43:146-154. [72] ZHAO B, PENG Q, WANG D, et al. Leonurine Protects Bone Mesenchymal Stem Cells from Oxidative Stress by Activating Mitophagy through PI3K/Akt/mTOR Pathway. Cells. 2022;11:1724. [73] JIANG SD, YANG YH, CHEN JW, et al. Isolated osteoblasts from spinal cord-injured rats respond less to mechanical loading as compared with those from hindlimb-immobilized rats. J Spinal Cord Med. 2013;36: 220-224. [74] YANG X, JIANG T, WANG Y, et al. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Sci Rep. 2019;9:18424. [75] COLE S, GIANFERANTE DM, ZHU B, et al. Osteosarcoma: A Surveillance, Epidemiology, and End Results program-based analysis from 1975 to 2017. Cancer. 2022;128:2107-2118. [76] WHELAN JS, DAVIS LE. Osteosarcoma, Chondrosarcoma, and Chordoma. J Clin Oncol. 2018;36:188-193. [77] DENG Z, BI S, JIANG M, et al. Endogenous H (2) S-Activated Orthogonal Second Near-Infrared Emissive Nanoprobe for In Situ Ratiometric Fluorescence Imaging of Metformin-Induced Liver Injury. ACS Nano. 2021;15:3201-3211. [78] WEIL R, LAPLANTINE E, CURIC S, et al. Role of Optineurin in the Mitochondrial Dysfunction: Potential Implications in Neurodegenerative Diseases and Cancer. Front Immunol. 2018;9:1243. [79] ICARD P, LINCET H. A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim Et Biophys Acta. 2012;1826:423-433. [80] GIACOMELLO M, PYAKUREL A, GLYTSOU C, et al. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020;21: 204-224. [81] ICHIM G, LOPEZ J, AHMED SU, et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol Cell. 2015;57:860-872. [82] DAS CK, MANDAL M, KÖGEL D. Pro-survival autophagy and cancer cell resistance to therapy. Cancer Metastasis Rev. 2018;37:749-766. [83] MEI L, SANG W, CUI K, et al. Norcantharidin inhibits proliferation and promotes apoptosis via c-Met/Akt/mTOR pathway in human osteosarcoma cells. Cancer Sci. 2019;110:582-595. [84] YANG C, YANG QO, KONG QJ, et al. Parthenolide Induces Reactive Oxygen Species-Mediated Autophagic Cell Death in Human Osteosarcoma Cells. Cell Physiol Biochem. 2016;40:146-154. [85] VIANELLO C, COCETTA V, CATANZARO D, et al. Cisplatin resistance can be curtailed by blunting Bnip3-mediated mitochondrial autophagy.Cell Death Dis. 2022;13:398. [86] HE G, PAN X, LIU X, et al. HIF-1-Mediated Mitophagy Determines ZnO Nanoparticle-Induced Human Osteosarcoma Cell Death both In Vitro and In Vivo. ACS Appl Mater Interfaces. 2020;12:48296-48309. [87] PAN X, HE G, HAI B, et al. VPS34 regulates dynamin to determine the endocytosis of mitochondria-targeted zinc oxide nanoparticles in human osteosarcoma cells. J Mater Chem B. 2021;9:2641-2655. [88] ZHANG L, JIAO G, YOU Y, et al. Arginine methylation of PPP1CA by CARM1 regulates glucose metabolism and affects osteogenic differentiation and osteoclastic differentiation. Clin Transl Med. 2023; 13(9):e1369. [89] HUANG D, PENG Y, LI Z, et al. Compression-induced senescence of nucleus pulposus cells by promoting mitophagy activation via the PINK1/PARKIN pathway. J Cell Mo. Med. 2020;24:5850-5864. [90] LAN T, ZHENG YC, LI ND, et al. CRISPR/dCas9-Mediated Parkin Inhibition Impairs Mitophagy and Aggravates Apoptosis of Rat Nucleus Pulposus Cells Under Oxidative Stress. Front Mol Biosci. 2021;8:674632. |
| [1] | Haonan Yang, Zhengwei Yuan, Junpeng Xu, Zhiqi Mao, Jianning Zhang. Preliminary study on the mechanisms and efficacy of deep brain stimulation in treating depression [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(在线): 1-9. |
| [2] | Wang Qisa, Lu Yuzheng, Han Xiufeng, Zhao Wenling, Shi Haitao, Xu Zhe. Cytocompatibility of 3D printed methyl acrylated hyaluronic acid/decellularized skin hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1912-1920. |
| [3] | Gao Yanguo, Guo Xu, Li Xiaohan, Chen Shiqi, Zhu Haitao, Huang Liangyong, Ye Fang, Lu Wei Wang Qibin, Zheng Tao, Chen Li. Optimization of prescription ratio of “Honghuangbai” gel by orthogonal test in diabetic skin wound mouse models [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1921-1928. |
| [4] | Liu Hongjie, Mu Qiuju, Shen Yuxue, Liang Fei, Zhu Lili. Metal organic framework/carboxymethyl chitosan-oxidized sodium alginate/platelet-rich plasma hydrogel promotes healing of diabetic infected wounds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1929-1939. |
| [5] | Min Changqin, Huang Ying. Construction of pH/near-infrared laser stimuli-responsive drug delivery system and its application in treatment of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1940-1951. |
| [6] | Shao Ziyu, Li Qian, Qumanguli·Abudukelimu, Han Youjun, Hu Yang. Preparation and characterization properties of three different ratios of biphasic calcium phosphate [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1952-1961. |
| [7] | Zheng Xuying, Hu Hongcheng, Xu Libing, Han Jianmin, Di Ping. Stress magnitude and distribution in two-piece cement-retained zirconia implants under different loading conditions and with varying internal connection shapes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1979-1987. |
| [8] | Zhou Hongli, Wang Xiaolong, Guo Rui, Yao Xuanxuan, Guo Ru, Zhou Xiongtao, He Xiangyi. Fabrication and characterization of nanohydroxyapatite/sodium alginate/polycaprolactone/alendronate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1962-1970. |
| [9] | Yang Lixia, Diao Liqin, Li Hua, Feng Yachan, Liu Xin, Yu Yuexin, Dou Xixi, Gu Huifeng, Xu Lanju. Regulatory mechanism of recombinant type III humanized collagen protein improving photoaging skin in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1988-2000. |
| [10] |
Dong Chunyang, Zhou Tianen, Mo Mengxue, Lyu Wenquan, Gao Ming, Zhu Ruikai, Gao Zhiwei.
Action mechanism of metformin combined with Eomecon chionantha Hance dressing in treatment of deep second-degree burn wounds#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2001-2013.
|
| [11] | Pan Zhiyi, Huang Jiawen, Xue Wenjun, Xu Jianda. Advantages of MXene-based flexible electronic sensors and their application in monitoring diabetic foot wounds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2023-2032. |
| [12] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [13] | Sun Lei, Zhang Qi, Zhang Yu. Pro-osteoblastic effect of chlorogenic acid protein microsphere/polycaprolactone electrospinning membrane [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1877-1884. |
| [14] | Wu Yanting, Li Yu, Liao Jinfeng. Magnesium oxide nanoparticles regulate osteogenesis- and angiogenesis-related gene expressions to promote bone defect healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1885-1895. |
| [15] | Li Qingbin, Lin Jianhui, Huang Wenjie, Wang Mingshuang, Du Jiankai, Lao Yongqiang. Bone cement filling after enlarged curettage of giant cell tumor around the knee joint: a comparison of subchondral bone grafting and non-grafting [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1896-1902. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||