Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (6): 1239-1247.doi: 10.12307/2025.299
Previous Articles Next Articles
ACSL4 mediates ferroptosis and its potential role in atherosclerotic cardiovascular disease
Gao Yang1, Qin Hewei1, 2, Liu Dandan1
- 1School of Rehabilitation, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; 2The Second Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2024-01-16Accepted:2024-03-27Online:2025-02-28Published:2024-06-22 -
Contact:Qin Hewei, MD, Associate chief physician, School of Rehabilitation, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; The Second Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
About author:Gao Yang, Master, School of Rehabilitation, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China -
Supported by:the National Natural Science Foundation of China, No. 82374551 (to QHW); Key Research Programs of Higher Education Institutions in Henan Province, No. 24B360003 (to QHW); Central Plains Talent Program for Young Top Talents, No. [2021]44 (to QHW); Henan Province Chinese Medicine Top Talent Training Program, No. [2021]15 (to QHW); Henan University of Chinese Medicine Postgraduate Research Innovation Program, No. 2023KYCX079 (to GY)
CLC Number:
Cite this article
Gao Yang, Qin Hewei, Liu Dandan. ACSL4 mediates ferroptosis and its potential role in atherosclerotic cardiovascular disease[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1239-1247.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
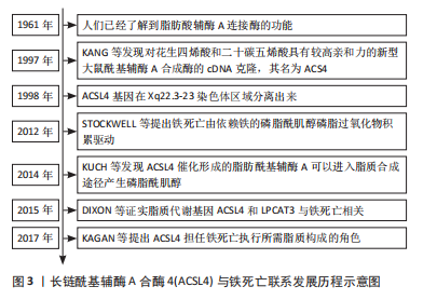
2.1 ACSL4的概述 2.1.1 ACSL4的发展历程 长链脂肪酰基辅酶A合成酶是将游离脂肪酸转换为酰基辅酶A形式的酶家族。1960年以来,人们已经了解到脂肪酸辅酶A连接酶的功能[6]。随着基因技术的发展,科学家们开始发现脂肪酸辅酶A连接酶的多样性[7]。之后,ACSL4基因于1998年首次被研究人员在患有Alport综合征、椭圆细胞增多症和智力低下患者的Xq22.3-23染色体区域分离出来[8]。随后的研究表明,ACSL4的生物学功能是将辅酶A插入多不饱和脂肪酸中来催化脂肪酰基辅酶A的形成,由于脂肪酸进入大多数代谢途径之前必须转化为酰基辅酶A,因此ACSL4的激活可有效促进脂质代谢进程[9],进一步研究发现,催化形成的脂肪酰基辅酶A可以进入脂质合成途径产生磷脂酰肌醇[10]。而2012年由STOCKWELL最早提出的铁死亡定义中显示,铁死亡由依赖铁的磷脂酰肌醇磷脂过氧化物积累驱动[11]。为证实ACSL4与铁死亡是否有联系,DIXON等[12]用Erastin、RSL3 和 ML162 诱导KBM7细胞铁死亡,以遗传基因学分析细胞鉴定出基因ACSL4和溶血卵磷脂酰基转移酶3,同时发现经历铁死亡的细胞缺乏花生四烯酸和其他多不饱和脂肪酸,揭示脂质代谢基因ACSL4与铁死亡相关,这项研究打开了ACSL4介导铁死亡的大门。此外,YUAN等[13]进一步研究为提出ACSL4不仅是铁死亡的敏感监测者,而且是铁死亡的重要贡献者提供直接证据。而ACSL4在铁死亡过程中担任怎样的角色呢?KAGAN等[14]发现花生四烯酸和肾上腺酸在ACSL4和其他酶的催化作用下形成参与铁死亡唯一脂质过氧化底物,揭示ACSL4对于铁死亡执行所需脂质构成至关重要。随着ACSL4与铁死亡的研究逐渐深入,ACSL4介导铁死亡在各类疾病中的作用机制逐渐被挖掘,成为研究热点。ACSL4的研究历程见图3。"

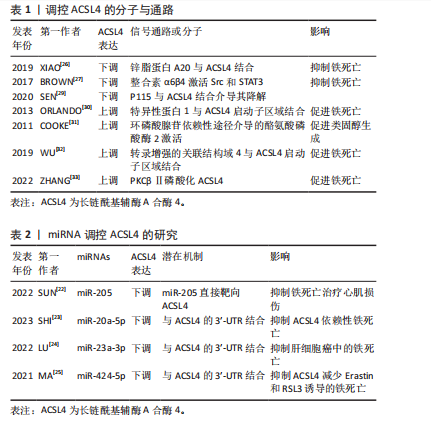
2.1.2 ACSL4的基因及蛋白结构 长链脂肪酰基辅酶A合成酶驱动游离的长链脂肪酸转化为脂肪酰基辅酶A酯,并在脂肪酸为细胞代谢和生长提供能量方面发挥主导作用[15]。ACSL4 基因编码酶倾向于20碳链的多不饱和脂肪酸,揭示ACSL4是多不饱和脂肪酸代谢的重要同工酶[16]。ACSL4基因位于Xq23染色体上,其cDNA编码区为2 136 bp,编码712个氨基酸残基[17]。此外,肾上腺、卵巢、睾丸、肝脏和脑组织等多种组织富含ACSL4,其蛋白结构由5个区域组成:氨基末端、荧光素酶样区域1和2、连接2个荧光素酶样区域的接头和羧基末端,而ACSL4缺乏50个与氨基对应的氨基酸,这可能是导致ACSL4对脂肪酸偏好不同的原因所在[18]。 2.1.3 ACSL4定位及生物学功能 ACSL4依赖性脂肪酸活化是脂质分子进入维持细胞代谢以产生能量和脂肪生成的必要先决条件,而ACSL4的亚细胞定位模式更接近内质网驻留伴侣钙连接蛋白的定位模式。研究发现,ACSL4富集在与线粒体形成紧密接触的内质网特殊区域,参与脂质合成和线粒体β氧化环节[19],其亚细胞定位也主要在分泌途径中的内体、过氧化物酶体和质膜中。ACSL4所需底物特异性在同工酶中显著不同,并偏好于长链多不饱和脂肪酸,后者发挥储存能量供应、形成细胞膜和提供信号转导以及蛋白质相互作用的功能。进一步研究发现,ACSL4通过激活脂肪酸并将辅酶A基团添加到脂肪酸中转化为脂肪酰基辅酶A酯,有效地捕获细胞中的脂肪酸[20],而生成的脂肪酰基辅酶A酯在溶血卵磷脂酰基转移酶3催化作用下与磷脂酰乙醇胺反应生成花生四烯酸/肾上腺酸-磷脂酰乙醇胺,然后通过铁离子、15-脂氧合酶氧化以产生有害的脂质氢过氧化物,从而诱导细胞铁死亡[21]。由上可知,ACSL4在脂肪酸代谢中发挥关键作用,而且是调控铁死亡发生的关键酶。 2.1.4 ACSL4的调控机制 随着ACSL4的研究成为热点,通过正向或负向调控其表达,促进或抑制ACSL4介导脂质氧化底物形成引发铁死亡的发生已成为潜在靶标。一方面,ACSL4的表达受到miR-205、miR-20a-5p、miR-23a-3p、miR-424-5p等负调控[22-25],大部分通过靶向ACSL4 mRNA的3’非翻译区域抑制其表达。同时研究发现,miRNA-17-92靶向A20/ACSL4轴抑制Erastin诱导的内皮细胞铁死亡,潜在机制为内皮细胞铁死亡的新型调节因子锌脂蛋白A20与ACSL4直接相互作用下调ACSL4抑制铁死亡[26]。现已确定几种抑制ACSL4的分子,如α6β4整合素介导信号传导和转录激活因子3的激活抑制ACSL4的表达,缺乏α6β4的贴壁细胞不能正常诱导铁死亡,原因在于ACSL4表达升高的同时,铁死亡抵御系统中谷胱甘肽过氧化酶4(glutathione peroxidase 4,GPX4)持续表达,从而避免细胞凋亡[27]。然而,乙酰化信号传导和转录激活因子3可上调ACSL4的表达,从而增加细胞膜和线粒体膜磷脂合成[28]。值得注意的是,花生四烯酸可能促进ACSL4泛素化和蛋白酶降解而下调ACSL4表达。最近研究发现,细胞内囊泡运输蛋白p115与ACSL4高亲和力结合并降解ACSL4,而花生四烯酸处理细胞与p115表达降低的联合结果表明p115在AA处理细胞导致的ACSL4降解具有催化作用[29]。 另一方面,ACSL4的表达上调被证明可通过特异性蛋白1、环磷酸腺苷反应原件结合、蛋白激酶C βⅡ和酪氨酸磷酸酶2正向调控。研究表明,ACSL4启动子的序列分析显示其中存在特异性蛋白1和环磷酸腺苷反应元件结合位点等转录因子结合位点,近端启动子中特殊蛋白1结合位点参与基础活性,而环磷酸腺苷反应元件结合中环磷酸腺苷刺激ACSL4转录增加其含量[30]。此外,含src同源性的酪氨酸磷酸酶2通过环磷酸腺苷依赖性途径激活上调ACSL4 mRNA和蛋白质表达,但具体机制尚不明确[31]。 转录增强的关联结构域 4与ACSL4启动子区结合并上调ACSL4转录表达,而启动子可以通过其共激活因子Yes相关蛋白增强[32],上述研究表明ACSL4表达可以通过某些转录因子转录增加。此外,ZHANG等[33]研究发现蛋白激酶C βⅡ作为脂质过氧化传感器,活化的蛋白激酶C βⅡ直接与ACSL4的Thr328处相互作用,磷酸化和激活ACSL4从而促进含多不饱和脂肪酸脂质的生物合成,诱导铁死亡。虽然越来越多的研究集中在ACSL4上,对于ACSL4的调控机制得到深入研究,但仍需进一步探究其详细机制。见表1,2。 2.2 铁死亡中的ACSL4 铁死亡归因于各种氧化应激诱导的脂质过氧化物铁依赖性积累,是一种非凋亡程序性细胞死亡形式。铁死亡与焦亡、凋亡、自噬的细胞形态学、生物学特性和遗传学存在明显差异,在形态学上,铁死亡伴随质膜破裂、染色质凝聚和线粒体外膜破裂、线粒体萎缩、膜密度增加以及嵴减少或缺失;在生化方面,铁积蓄通过芬顿反应产生大量活性氧引起脂质过氧化和细胞内铁积累是关键生化特征[34]。铁死亡处于铁、不饱和脂肪酸和谷胱甘肽等生物代谢过程交汇处,由复杂机制调控铁死亡激活和抵御系统[35]。然而,有研究表明,铁代谢、线粒体电子传递链和烟酰胺腺嘌呤二核苷酸磷酸氧化酶家族来源的活性氧是铁死亡的初始信号,而ACSL4"
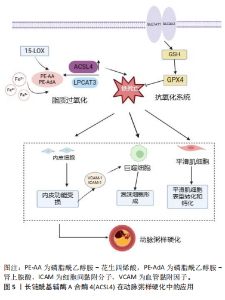
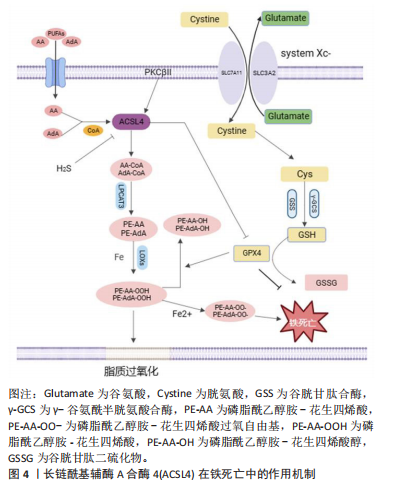
2.2.1 ACSL4介导脂质过氧化调控铁死亡 脂质过氧化是引发铁死亡的主要信号。细胞膜中含有多不饱和脂肪酸的磷脂与自由基相互作用引发细胞膜、细胞器膜和线粒体发生脂质过氧化导致细胞铁死亡,而将多不饱和脂肪酸掺入磷脂中需要ACSL4参与,具体机制为ACSL4和溶血卵磷脂酰基转移酶3协同酯化花生四烯酸和肾上腺酸为相应的磷脂酰乙醇胺,然后在富含铁和氧的环境被氧化生成磷脂酰乙醇胺-花生四烯酸氢过氧化物和磷脂酰乙醇胺-肾上腺酸氢过氧化物[14]。研究表明,ACSL4的磷酸化和激活,放大脂质过氧化促进脂质氢过氧化物合成达到铁死亡危险水平[33]。进一步研究显示,特异性敲低ACSL4的表达可有效减少其介导的脂质过氧化底物生成,从而抑制Erastin诱导的铁死亡[13],TOMITSUKA等[38]研究证实了这一观点,小鼠肺中ACSL4表达下调能有效减轻肺毒性诱导的肺纤维化,潜在机制为ACSL4基因缺失减少脂质过氧化底物含多不饱和脂肪酸的磷脂生成,进而抑制ACSL4介导的铁死亡。此外,铁死亡激动剂RSL3干预导致多不饱和脂肪酸过氧化物不能被及时清除而蓄积从而诱发细胞铁死亡,而胱硫硫氨酸γ裂解酶衍生的硫化氢逆转铁死亡激动剂RSL3诱导的ACSL4表达上调,同时减弱膜磷脂氧化关键酶ALOX12的乙酰化阻止过氧化物生成进而阻止铁死亡[39]。以上研究皆证实ACSL4表达是铁死亡的重要生物学因子,ACSL4过表达促进脂质过氧化底物生成是诱导铁死亡的基础且不可或缺。越来越多研究表明ACSL4蛋白可作为铁死亡疾病治疗的潜在靶点,但具体机制仍需进一步探究。 2.2.2 ACSL4 影响GPX4调控铁死亡 铁死亡是由于脂质过氧化的细胞代谢与细胞抗氧化系统之间的拮抗作用失衡导致的调节性坏死细胞死亡形式。铁死亡的抗氧化防御系统包括谷胱甘肽、硒和辅酶Q,而质膜上胱氨酸/谷氨酸反向转运体介导的胱氨酸摄取和随后的谷胱甘肽产生和GPX4活化在保护细胞免受铁死亡中起着核心作用。GPX4作为抗氧化系统的重要活性酶,谷胱甘肽是GPX4解毒脂质氢过氧化物抑制铁死亡的重要辅助因子,在GPX4的催化循环中,谷胱甘肽作为GPX4底物被氧化为氧化型谷胱甘肽,同时谷胱甘肽还原硒酸保持GPX4活性从而催化毒性脂质过氧化物还原为脂质醇抑制铁死亡[40],而氨基酸代谢紊乱使谷胱甘肽合成减少,GPX4活性降低导致脂质氢过氧化物积累破坏膜结构从而增加铁死亡敏感性[41]。因此,提高GPX4活性,促进谷胱甘肽生成可抑制铁死亡。 ACSL4作为铁死亡的特异性敏感因子,介导脂质过氧化底物生成调控铁死亡。有研究显示,ACSL4与抑制GPX4活性引发铁死亡密切相关且二者相互影响,ACSL4与GPX4之间的关系一直是关于铁死亡研究的重要领域。以前研究表明,ACSL4 在某些情况下诱导的铁死亡中具有可有可无的作用,例如 p53 介导的铁死亡[42]。但INGOLD等[43]研究发现,在高表达GPX4的细胞中ACSL4的表达下调,从而降低铁死亡信号,而进一步敲除ACSL4使高表达GPX4细胞对过氧化物诱导的细胞死亡更具抵抗力,揭示ACSL4参与GPX4抑制铁死亡的调节过程。最近,MAGTANONG等[44]发现在RSL3与Erastin诱导的铁死亡中,ACSL4的需求存在显著差异,并在几种细胞系中证实了 ACSL4 缺陷细胞对 GPX4 抑制剂诱导的铁死亡的抵抗力确实比对胱氨酸耗竭触发的铁死亡更具抵抗力,表明ACSL4对于直接抑制GPX4活性执行铁死亡比胱氨酸缺失更为重要。因此,这些研究阐明ACSL4与GPX4二者互为铁死亡负调控因子,相辅相成,可为铁死亡调节提供理论基础,但其具体机制仍待进一步研究。 2.3 ACSL4在动脉粥样硬化性心血管疾病中的作用 2.3.1 ACSL4在动脉粥样硬化中的应用 近年来,有研究报道铁死亡促进动脉粥样硬化病变的形成。内皮细胞炎症激活和功能障碍是动脉粥样硬化开始的关键事件。在内皮细胞稳态失衡后,其上调E-选择素、细胞间黏附分子和血管细胞黏附分子加剧组织炎症增加内皮通透性,使脂蛋白颗粒在内皮下间隙的渗透加速脂质沉积[45]。铁超载通过芬顿反应产生大量活性氧引起氧化应激,同时上调细胞间黏附分子和血管细胞黏附分子1表达、降低一氧化氮生物利用度损伤内皮细胞,进而促进内皮下脂蛋白浸润和单核细胞募集加快斑块脂质沉积[46]。铁死亡抑制剂Ferrostatin-1能够减轻ApoE小鼠主动脉内皮细胞的脂质过氧化和内皮功能障碍来减轻动脉粥样硬化。ZHANG等[47]以氧化低密度脂蛋白构建内皮损伤模型发现,细胞中铁含量、ACSL4增加,而谷胱甘肽和GPX4含量降低促进铁死亡,且同时体内实验发现小鼠脂质沉积,斑块数量及大小增加,以上研究证明铁死亡参与动脉粥样硬化的发生,ACSL4参与脂质过氧化诱导铁死亡是内皮细胞损伤的关键。进一步探究铁死亡相关蛋白表达与动脉粥样硬化严重程度之间的相关性发现,ACSL4表达明显上调且与动脉粥样硬化严重程度呈正相关[48],有直接证据证实,Erastin诱导铁死亡发生损伤血管内皮细胞与ACSL4表达上调密切相关,随后经miR-17-92过表达靶向ACSL4并下调其表达后,可有效减少脂质过氧化逆转内皮细胞功能障碍[26], 揭示ACSL4调控铁死亡敏感性,并可通过抑制其表达保护内皮功能并减少脂质沉积。此外,铁沉积触发巨噬细胞源性泡沫细胞形成和血管平滑肌细胞表型转化[49-50],铁死亡的发生进一步加速平滑肌细胞钙化导致斑块稳定性降低,YE等[51]发现在高钙/磷酸盐诱导的血管平滑肌细胞钙化过程中,脂质活性氧显著升高,xCT、谷胱甘肽和GPX4表达降低。体外抑制铁死亡抑制了成骨条件下血管平滑肌细胞的钙化,并减轻动脉环钙化。因此,铁死亡通过多途径参与动脉粥样硬化斑块形成和发展,见图5,ACSL4可能是抑制铁死亡改善动脉粥样硬化内皮损伤的潜在靶点,而有关ACSL4 在泡沫细胞形成和血管平滑肌细胞中相关研究尚未见到。因此,ACSL4介导铁死亡与动脉粥样硬化病理机制的相关性仍需进一步探索。 2.3.2 缺血性脑卒中的ACSL4 颈动脉中动脉粥样硬化斑块破裂引起的血栓栓塞事件导致缺血性脑卒中,它是全球第二大死亡原因。目前,尽快恢复缺血区血液仍是缺血性脑卒中主要治疗策略,如药物溶栓、机械血管内血栓切除术,但预后较差[52]。再灌注后铁代谢紊乱,铁超载通过芬顿反应产生氧自由基促进脂质过氧化激活铁死亡[53],同时由于脑缺血再灌注期间ATP产生不足导致系统Xc?胱"
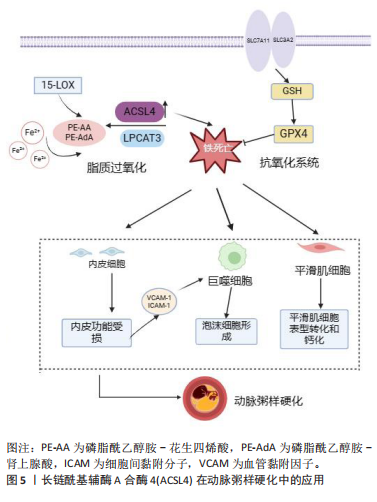

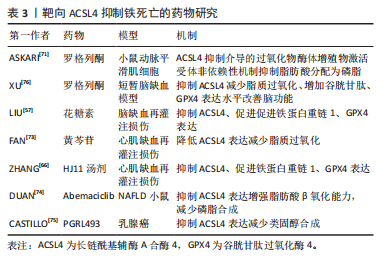
氨酸/谷氨酸逆向转运蛋白的活性受到抑制,抗氧化剂谷胱甘肽产生减少、GPX4活性降低加剧铁死亡。研究表明抑制脂质过氧化和减少谷胱甘肽靶向铁死亡是改善卒中后脑功能的有效靶点[54]。 铁死亡抑制剂ferrostatin-1可缓解再灌注后脑损伤和神经功能缺损。脂质过氧化通过破坏蛋白质、DNA和脂质膜激活铁死亡攻击细胞,ACSL4是一种诱导脂质过氧化和铁死亡的关键脂质代谢酶。为观察局灶性缺血后ASCL4表达变化,CUI等[55]以小鼠构建瞬时大脑中动脉闭塞模型为研究对象,在缺血1-3 h后ACSL4 在同侧皮质中的表达显著降低,复氧 6 h后升高至基础水平,且ACSL4表达在卒中早期的抑制是由缺氧诱导因子1α介导,进一步转染ACSL4过表达发现其通过增强脂质过氧化促进神经元死亡,相反敲低ACSL4可减轻小鼠脑损伤。TUO等[56]研究发现,在缺血再灌注期间,ACSL4的减少可能是翻译后修饰的结果,同时表明凝血酶诱导铁死亡,ACSL4可以介导凝血酶细胞毒性,而ACSL4抑制剂吡格列酮可以阻断凝血酶细胞毒性。这些结果表明,ACSL4的减少可能有助于抑制凝血酶诱导的铁死亡,而ACSL4为参与缺血再灌注相关细胞铁死亡的关键蛋白。最近有研究显示,以花糖素干预缺血再灌注大鼠模型可降低ACSL4表达,上调GPX4活性从而抑制ACSL4依赖性铁死亡改善脑损伤[57],进一步研究发现,通过抑制特异性蛋白1靶向降低ACSL4水平,可有效抑制铁死亡抗脑损伤,内在机制为特异性蛋白1与ACSL4启动子区域结合可增加后者表达,证实抑制ACSL4是抑制铁死亡的有效靶标[58]。 2.3.3 心肌梗死-缺血再灌注中的ACSL4 心肌梗死是全球最常见的死亡原因,由动脉壁斑块形成引起冠状动脉血流长期中断,从而导致严重的心肌组织缺血和心肌细胞损伤[59]。目前,干预心肌梗死最有效策略是及时溶栓治疗和初次经皮冠状动脉介入治疗,在快速恢复血液循环的同时限制心肌梗死面积和避免心肌衰竭[60],但也会引起氧化应激损伤、炎症和细胞死亡。缺血性心肌再灌注后的活性氧积累和铁超负荷是铁死亡主要特征,最近有研究证实,铁死亡是心肌梗死的主要因素,ACSL4在铁死亡过程中发挥重要作用[61]。 既往研究表明,大多数ST段抬高型心肌梗死患者心肌残留铁导致铁死亡从而影响左心室重塑[62]。最近一项研究显示,脂质过氧化关键酶ACSL4的表达在ST段抬高型心肌梗死患者的血清中升高,且与预后良好组相比,预后不良组血清ACSL4、白细胞介素1β水平显著升高[63]。YU等[64]构建小鼠急性心肌梗死模型研究发现,模型组小鼠ACSL4水平、丙二醛、铁含量更高,GPX4表达下降,而给药后逆转此变化,有效抑制铁死亡改善心肌缺血性损伤,同时增加缺血性心肌中miR-450b-5p的表达,基于此,转染miR-450b-5p 和 ACSL4 沉默或过表达质粒探究其内在机制发现,过表达 miR-450b-5p 可下调 ACSL4表达,和miR-450b-5p抑制剂具有相反的效果,揭示靶向miR-450b-5p抑制铁死亡和减轻心肌缺血与调控ACSL4表达密切相关。然而,有研究显示,铁死亡主要发生在心肌再灌注期,心肌组织缺血区铁死亡相关因子ACSL4、铁和丙二醛以及GPX4表达没有显著变化[65],因此,铁死亡与心肌梗死-缺血再灌注的关系仍待研究。脂质过氧化作为铁死亡发生发展的关键组成部分,被证明是导致缺血再灌注氧化损伤的重要因素。在氧葡萄糖剥夺/再灌注诱导的H9c2细胞模型中,ACSL4过表达触发氧化应激和铁死亡加重心肌损伤,敲低ACSL4可减弱细胞中铁积累和氧化应激改善心肌功能[66]。近期,QIU等[67]发现YAP在抑制心肌缺血再灌注后ACSL4诱导的心肌细胞铁死亡发挥关键作用,进一步研究表明内在机制为YAP促进神经前体细胞表达发育下调4蛋白转录,从而导致ACSL4的泛素化和降解,抑制铁死亡发生改善心脏功能。综上所述,铁死亡影响心肌梗死-缺血再灌注损伤进展,而调节ACSL4的表达是抑制铁死亡改善心功能的潜在靶标。 2.4 ACSL4作为铁死亡的生物标志物和潜在靶点 目前,抑制铁死亡的主要方法分为针对铁沉积和脂质过氧化的两种药物治疗。铁螯合剂作为铁死亡抑制剂,可通过靶向铁转运蛋白调控铁代谢限制铁沉积、降低线粒体活性氧生成等途径改善铁死亡,去铁胺、地拉罗司和去铁酮是FDA批准的3种螯合剂。另外,抗氧化剂他汀类药物、维生素E和溶血磷脂酸盐通过防止自由基形成、调节GPX4、ACSL4表达和辅酶Q10生物合成阻止过氧化过程间接抑制铁死亡[68]。 基于动物和分子水平的研究,血液中铁、脂质过氧化物和转铁蛋白的基因和蛋白被作为铁死亡标志物。最近研究发现,在铁死亡敏感细胞中,ACSL4表达相比于铁死亡抵抗细胞显著上调,且ACSL4介导的5-羟基二十四烯酸产生有助于铁死亡发生,证明ACSL4可以被作为铁死亡标志物,且是铁死亡发生的重要贡献者[13]。为证明ACSL4作为铁死亡生物标志物的更有助于诊断及预测疾病预后情况。SHA等[69]通过纳入199例乳腺癌患者探究铁死亡相关基因ACSL4和GPX4作为接受新辅助化疗的乳腺癌患者预后生物标志物的关键价值,经分析表明,ACSL4在总生存期长的患者中表达上调,而GPX4高表达则与远处无转移有关,揭示ACSL4和GPX4的表达强弱可能预测患者的生存情况。此外,由上文可知ACSL4与铁死亡发生过程密切相关,其可作为铁死亡疾病治疗的潜在靶点,见表3。近年来,靶向ACSL4的抑制剂包括FDA批准的药物罗格列酮和吡格列酮,其可阻止脂质过氧化诱导铁死亡的发生[70]。有研究显示,罗格列酮选择性抑制ACSL4从而降低平滑肌细胞和巨噬细胞中脂肪酸分配为磷脂的能力,推测可能通过减少含AA/AdA-磷脂酰乙醇胺的亚铁前体AA和AdA抑制铁死亡减缓动脉粥样硬化的发展[71]。此外,CHEN等[72]在大脑中动脉闭塞小鼠中建立了短暂性脑缺血模型,在大脑中动脉闭塞前1 h静脉注射罗格列酮,用罗格列酮抑制ACSL4后,GPX4的降低明显减弱,脑卒中后72 h神经功能明显改善,脑梗死体积减少。除此之外,抑制ACSL4介导的铁死亡改善心血管疾病的中药研究逐渐被发掘。黄芩素和HJ11汤剂都通过抑制促铁死亡蛋白ACSL4表达,促进抗氧化因子GPX4表达调节铁死亡,改善心脏功能[66,73]。然而,有研究抑制ACSL4的新化合物有Abemaciclib和PGRL493[74-75],前者直接抑制ACSL4表达增强脂肪酸β氧化并减少脂质积累降低铁死亡敏感性以改善疾病病程,因此,靶向抑制ACSL4为动脉粥样硬化性心血管疾病治疗提供了合理的理论基础。见表3。总之,ACSL4可能作为判断铁死亡发生的新型生物标志物,且作为潜在靶标抑制铁死亡损伤,但靶向ACSL4的药物种类不多,揭示未来靶向ACSL4抑制铁死亡改善疾病进程具有广阔研究前景,但具有挑战性[76]。 "
| [1] LIN L, ZHANG MX, ZHANG L, et al. Autophagy, Pyroptosis, and Ferroptosis: New Regulatory Mechanisms for Atherosclerosis. Front Cell Dev Biol. 2021;9:809955. [2] FAN J, WATANABE T. Atherosclerosis: Known and unknown. Pathol Int. 2022;72(3):151-160. [3] WU G, YU G, ZHENG M, et al. Recent Advances for Dynamic-Based Therapy of Atherosclerosis. Int J Nanomedicine. 2023;18:3851-3878. [4] XIN S, SCHICK JA. PUFAs dictate the balance of power in ferroptosis. Cell Calcium. 2023;110:102703. [5] BAI T, LI M, LIU Y, et al. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med. 2020;160:92-102. [6] HATCH MD, STUMPF PK. Fat metabolism in higher plants. XVI. Acetyl coenzyme A carboxylase and acyl coenzyme A-malonyl coenzyme A transcarboxylase from wheat germ. J Biol Chem. 1961;236:2879-2885. [7] KANG MJ, FUJINO T, SASANO H, et al. A novel arachidonate-preferring acyl-CoA synthetase is present in steroidogenic cells of the rat adrenal, ovary, and testis. Proc Natl Acad Sci U S A. 1997;94(7):2880-2884. [8] PICCINI M, VITELLI F, BRUTTINI M, et al. FACL4, a new gene encoding long-chain acyl-CoA synthetase 4, is deleted in a family with Alport syndrome, elliptocytosis, and mental retardation. Genomics. 1998; 47(3):350-358. [9] MASHEK DG, LI LO, COLEMAN RA. Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2007;2(4):465-476. [10] KÜCH EM, VELLARAMKALAYIL R, ZHANG I, et al. Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol. Biochim Biophys Acta. 2014;1841(2):227-239. [11] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5): 1060-1072. [12] DIXON SJ, WINTER GE, MUSAVI LS, et al. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem Biol. 2015;10(7):1604-1609. [13] YUAN H, LI X, ZHANG X, et al. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016; 478(3):1338-1343. [14] KAGAN VE, MAO G, QU F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017; 13(1): 81-90. [15] CHEN J, DING C, CHEN Y, et al. ACSL4 reprograms fatty acid metabolism in hepatocellular carcinoma via c-Myc/SREBP1 pathway. Cancer Lett. 2021;502:154-165. [16] KUWATA H, HARA S. Role of acyl-CoA synthetase ACSL4 in arachidonic acid metabolism. Prostaglandins Other Lipid Mediat. 2019;144:106363. [17] WU Z, SUN J, LIAO Z, et al. An update on the therapeutic implications of long-chain acyl-coenzyme A synthetases in nervous system diseases. Front Neurosci. 2022;16:1030512. [18] HOU J, JIANG C, WEN X, et al. ACSL4 as a Potential Target and Biomarker for Anticancer: From Molecular Mechanisms to Clinical Therapeutics. Front Pharmacol. 2022;13:949863. [19] RADIF Y, NDIAYE H, KALANTZI V, et al. The endogenous subcellular localisations of the long chain fatty acid-activating enzymes ACSL3 and ACSL4 in sarcoma and breast cancer cells. Mol Cell Biochem. 2018; 448(1-2):275-286. [20] KILLION EA, REEVES AR, EL AZZOUNY MA, et al. A role for long-chain acyl-CoA synthetase-4 (ACSL4) in diet-induced phospholipid remodeling and obesity-associated adipocyte dysfunction. Mol Metab. 2018;9:43-56. [21] SINGH AB, KAN CFK, KRAEMER FB, et al. Liver-specific knockdown of long-chain acyl-CoA synthetase 4 reveals its key role in VLDL-TG metabolism and phospholipid synthesis in mice fed a high-fat diet. Am J Physiol Endocrinol Metab. 2019;316(5):E880-E894. [22] SUN W, WU X, YU P, et al. LncAABR07025387.1 Enhances Myocardial Ischemia/Reperfusion Injury Via miR-205/ACSL4-Mediated Ferroptosis. Front Cell Dev Biol. 2022;10:672391. [23] SHI L, SONG Z, LI Y, et al. MiR-20a-5p alleviates kidney ischemia/reperfusion injury by targeting ACSL4-dependent ferroptosis. Am J Transplant. 2023;23(1):11-25. [24] LU Y, CHAN YT, TAN HY, et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41(1):3. [25] MA LL, LIANG L, ZHOU D, et al. Tumor suppressor miR-424-5p abrogates ferroptosis in ovarian cancer through targeting ACSL4. Neoplasma. 2021;68(1):165-173. [26] XIAO FJ, ZHANG D, WU Y, et al. miRNA-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the A20-ACSL4 axis. Biochem Biophys Res Commun. 2019;515(3):448-454. [27] BROWN CW, AMANTE JJ, GOEL HL, et al. The α6β4 integrin promotes resistance to ferroptosis. J Cell Biol. 2017;216(12):4287-4297. [28] LI YJ, FAHRMANN JF, AFTABIZADEH M, et al. Fatty acid oxidation protects cancer cells from apoptosis by increasing mitochondrial membrane lipids. Cell Rep. 2022;39(9):110870. [29] SEN P, KAN CFK, SINGH AB, et al. Identification of p115 as a novel ACSL4 interacting protein and its role in regulating ACSL4 degradation. J Proteomics. 2020;229:103926. [30] ORLANDO U, COOKE M, CORNEJO MACIEL F, et al. Characterization of the mouse promoter region of the acyl-CoA synthetase 4 gene: role of Sp1 and CREB. Mol Cell Endocrinol. 2013;369(1-2):15-26. [31] COOKE M, ORLANDO U, MALOBERTI P, et al. Tyrosine phosphatase SHP2 regulates the expression of acyl-CoA synthetase ACSL4. J Lipid Res. 2011;52(11):1936-1948. [32] WU J, MINIKES AM, GAO M, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572(7769): 402-406. [33] ZHANG HL, HU BX, LI ZL, et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol. 2022;24(1):88-98. [34] CHEN X, LI J, KANG R, et al. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054-2081. [35] STOCKWELL BR, FRIEDMANN ANGELI JP, BAYIR H, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171(2):273-285. [36] LIU J, KANG R, TANG D. Signaling pathways and defense mechanisms of ferroptosis. Febs J. 2022;289(22):7038-7050. [37] DOLL S, PRONETH B, TYURINA YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017; 13(1):91-98. [38] TOMITSUKA Y, IMAEDA H, ITO H, et al. Gene deletion of long-chain acyl-CoA synthetase 4 attenuates xenobiotic chemical-induced lung injury via the suppression of lipid peroxidation. Redox Biol. 2023;66:102850. [39] WANG Y, YU R, WU L, et al. Hydrogen sulfide guards myoblasts from ferroptosis by inhibiting ALOX12 acetylation. Cell Signal. 2021; 78:109870. [40] LI FJ, LONG HZ, ZHOU ZW, et al. System X(c) (-)/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front Pharmacol. 2022;13:910292. [41] WEI Y, LV H, SHAIKH AB, et al. Directly targeting glutathione peroxidase 4 may be more effective than disrupting glutathione on ferroptosis-based cancer therapy. Biochim Biophys Acta Gen Subj. 2020;1864(4): 129539. [42] CHU B, KON N, CHEN D, et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21(5):579-591. [43] INGOLD I, BERNDT C, SCHMITT S, et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. 2018; 172(3):409-422.e421. [44] MAGTANONG L, MUELLER GD, WILLIAMS KJ, et al. Context-dependent regulation of ferroptosis sensitivity. Cell Chem Biol. 2022;29(9): 1409-1418.e1406. [45] MUSSBACHER M, SCHOSSLEITNER K, KRAL-POINTNER JB, et al. More than Just a Monolayer: the Multifaceted Role of Endothelial Cells in the Pathophysiology of Atherosclerosis. Curr Atheroscler Rep. 2022;24(6): 483-492. [46] VINCHI F, PORTO G, SIMMELBAUER A, et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur Heart J. 2020;41(28):2681-2695. [47] ZHANG M, YU Z, ZHAO L, et al. Long non-coding RNA PVT1 regulates atherosclerosis progression via the microRNA-106b-5p/ACSL4 axis. Biochem Biophys Res Commun. 2023;667:170-179. [48] ZHOU Y, ZHOU H, HUA L, et al. Verification of ferroptosis and pyroptosis and identification of PTGS2 as the hub gene in human coronary artery atherosclerosis. Free Radic Biol Med. 2021;171:55-68. [49] LIU X, WU J, TIAN R, et al. Targeting foam cell formation and macrophage polarization in atherosclerosis: The Therapeutic potential of rhubarb. Biomed Pharmacother. 2020;129:110433. [50] YANG Z, SHI J, CHEN L, et al. Role of Pyroptosis and Ferroptosis in the Progression of Atherosclerotic Plaques. Front Cell Dev Biol. 2022;10:811196. [51] YE Y, CHEN A, LI L, et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. 2022; 102(6):1259-1275. [52] SU EJ, LAWRENCE DA. Diabetes and the treatment of ischemic stroke. J Diabetes Complications. 2022;36(11):108318. [53] XU Y, LI K, ZHAO Y, et al. Role of Ferroptosis in Stroke. Cell Mol Neurobiol. 2023;43(1):205-222. [54] WANG P, CUI Y, REN Q, et al. Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 2021;12(5):447. [55] CUI Y, ZHANG Y, ZHAO X, et al. ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun. 2021;93:312-321. [56] TUO Q Z, LIU Y, XIANG Z, et al. Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduct Target Ther. 2022;7(1):59. [57] LIU H, ZHAO Z, YAN M, et al. Calycosin decreases cerebral ischemia/reperfusion injury by suppressing ACSL4-dependent ferroptosis. Arch Biochem Biophys. 2023;734:109488. [58] LYU N, LI X. Sevoflurane Postconditioning Attenuates Cerebral Ischemia-Reperfusion Injury by Inhibiting SP1/ACSL4-Mediated Ferroptosis. Hum Exp Toxicol. 2023;42:9603271231160477. [59] XIE Y, WANG Y, ZHAO L, et al. Identification of potential biomarkers and immune cell infiltration in acute myocardial infarction (AMI) using bioinformatics strategy. Bioengineered. 2021;12(1):2890-2905. [60] KONIJNENBERG LSF, DAMMAN P, DUNCKER DJ, et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res. 2020;116(4):787-805. [61] ZHAO WK, ZHOU Y, XU TT, et al. Ferroptosis: Opportunities and Challenges in Myocardial Ischemia-Reperfusion Injury. Oxid Med Cell Longev. 2021;2021:9929687. [62] BULLUCK H, ROSMINI S, ABDEL-GADIR A, et al. Residual Myocardial Iron Following Intramyocardial Hemorrhage During the Convalescent Phase of Reperfused ST-Segment-Elevation Myocardial Infarction and Adverse Left Ventricular Remodeling. Circ Cardiovasc Imaging. 2016;9(10): e004940. [63] HU Y, LI Q, WANG Y. Serum ACSL4 levels in patients with ST-segment elevation myocardial infarction (STEMI) and its association with one-year major adverse cardiovascular events (MACE): A prospective cohort study. Medicine (Baltimore). 2024;103(2):e36870. [64] YU Q, ZHANG N, GAN X, et al. EGCG attenuated acute myocardial infarction by inhibiting ferroptosis via miR-450b-5p/ACSL4 axis. Phytomedicine. 2023;119:154999. [65] TANG LJ, LUO XJ, TU H, et al. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(2): 401-410. [66] ZHANG F, LI Z, GAO P, et al. HJ11 decoction restrains development of myocardial ischemia-reperfusion injury in rats by suppressing ACSL4-mediated ferroptosis. Front Pharmacol. 2022;13:1024292. [67] QIU M, YAN W, LIU M. YAP Facilitates NEDD4L-Mediated Ubiquitination and Degradation of ACSL4 to Alleviate Ferroptosis in Myocardial Ischemia-Reperfusion Injury. Can J Cardiol. 2023;39(11):1712-1727. [68] ZHANG Y, XIN L, XIANG M, et al. The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed Pharmacother. 2022;145:112423. [69] SHA R, XU Y, YUAN C, et al. Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. EBioMedicine. 2021;71:103560. [70] KUNG YA, CHIANG HJ, LI ML, et al. Acyl-Coenzyme A Synthetase Long-Chain Family Member 4 Is Involved in Viral Replication Organelle Formation and Facilitates Virus Replication via Ferroptosis. mBio. 2022; 13(1):e0271721. [71] ASKARI B, KANTER JE, SHERRID AM, et al. Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-gamma-independent mechanism in human arterial smooth muscle cells and macrophages. Diabetes. 2007;56(4):1143-1152. [72] CHEN J, YANG L, GENG L, et al. Inhibition of Acyl-CoA Synthetase Long-Chain Family Member 4 Facilitates Neurological Recovery After Stroke by Regulation Ferroptosis. Front Cell Neurosci. 2021;15:632354. [73] FAN Z, CAI L, WANG S, et al. Baicalin Prevents Myocardial Ischemia/Reperfusion Injury Through Inhibiting ACSL4 Mediated Ferroptosis. Front Pharmacol. 2021;12:628988. [74] DUAN J, WANG Z, DUAN R, et al. Therapeutic targeting of hepatic ACSL4 ameliorates NASH in mice. Hepatology. 2022;75(1):140-153. [75] CASTILLO AF, ORLANDO UD, MALOBERTI PM, et al. New inhibitor targeting Acyl-CoA synthetase 4 reduces breast and prostate tumor growth, therapeutic resistance and steroidogenesis. Cell Mol Life Sci, 2021;78(6):2893-2910. [76] XU Y, LI X, CHENG Y, et al. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. Faseb J. 2020;34(12): 16262-16275. |
| [1] | Wang Zheng, Cheng Ji, Yu Jinlong, Liu Wenhong, Wang Zhaohong, Zhou Luxing. Progress and future perspectives on the application of hydrogel materials in stroke therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2081-2090. |
| [2] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [3] | Wen Fan, Xiang Yang, Zhu Huan, Tuo Yanfang, Li Feng. Exercise improves microvascular function in patients with type 2 diabetes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1225-1235. |
| [4] | Zou Rongji, Yu Fangfang, Wang Maolin, Jia Zhuopeng. Triptolide inhibits ferroptosis and improves cerebral ischemia-reperfusion injury in a rat model of cerebral artery occlusion/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 873-881. |
| [5] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [6] | Yao Lijie, Yan Yuying, Chen Siyu, Wang Yuanfei, Wu Tong. Nanofibers with gradient deposition of endothelial cell derived matrix particles modulate the behavior of Schwann cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-10. |
| [7] | Wang Zheng, Cheng Ji, Yu Jinlong, Liu Wenhong, Wang Zhaohong, Zhou Luxing. Progress and future perspectives on the application of hydrogel materials in stroke therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-10. |
| [8] | Li Zikai, Zhang Chengcheng, Xiong Jiaying, Yang Xirui, Yang Jing, Shi Haishan. Potential effects of ornidazole on intracanal vascularization in endodontic regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-7. |
| [9] | Han Haihui, Ran Lei, Meng Xiaohui, Xin Pengfei, Xiang Zheng, Bian Yanqin, Shi Qi, Xiao Lianbo. Targeting fibroblast growth factor receptor 1 signaling to improve bone destruction in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1905-1912. |
| [10] | Jing Ruyi, Chen Yingxin, Cao Lei . Prognosis of deep lamellar keratoplasty versus penetrating keratoplasty in the treatment of stromal corneal dystrophy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1626-1633. |
| [11] | Zhao Nannan, Li Yanjie, Qin Hewei, Zhu Bochao, Ding Huimin, Xu Zhenhua. Changes in ferroptosis in hippocampal neurons of vascular dementia model rats treated with Tongmai Kaiqiao Pill [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1401-1407. |
| [12] | Zhang Mingyang, Yang Xinling. Verbascoside inhibits Erastin-induced ferroptosis of dopaminergic nerve cell line MN9D cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1408-1413. |
| [13] | Wan Lingling, Wu Mengying, Zhang Yujiao, Luo Qingqing. Inflammatory factor interferon-gamma affects migration and apoptosis of human vascular smooth muscle cells through pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1422-1428. |
| [14] | Wang Mi, Ma Shujie, Liu Yang, Qi Rui. Identification and validation of characterized gene NFE2L2 for ferroptosis in ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1466-1474. |
| [15] | Ba Yanhong, Gao Minghong, Chen Yingxin. Impact of graft thickness on corneal endothelial decompensation following simple Descemet’s stripping endothelial keratoplasty [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1199-1207. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||