Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (1): 156-163.doi: 10.12307/2024.724
Previous Articles Next Articles
Isolation technique and application of platelet-derived extracellular vesicles from platelet-rich plasma
Li Jiao1, Li Xiaofeng1, 2, Li Jianping1, 2, 3, 4
- 1Liaoning Institute of Transfusion Medicine, Liaoning Blood Center, Shenyang 110044, Liaoning Province, China; 2School of Pharmacy, China Medical University, Shenyang 110122, Liaoning Province, China; 3Harbin Blood Center, Harbin 150056, Heilongjiang Province, China; 4Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, Suzhou 215000, Jiangsu Province, China
-
Received:2023-08-29Accepted:2023-10-25Online:2025-01-08Published:2024-05-20 -
Contact:Li Jianping, MD, Chief physician, Liaoning Institute of Transfusion Medicine, Liaoning Blood Center, Shenyang 110044, Liaoning Province, China; School of Pharmacy, China Medical University, Shenyang 110122, Liaoning Province, China; Harbin Blood Center, Harbin 150056, Heilongjiang Province, China; Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, Suzhou 215000, Jiangsu Province, China Co-Corresponding author: Li Xiaofeng, Master, Chief technician, Liaoning Institute of Transfusion Medicine, Liaoning Blood Center, Shenyang 110044, Liaoning Province, China; School of Pharmacy, China Medical University, Shenyang 110122, Liaoning Province, China -
About author:Li Jiao, Master, Technician-in-charge, Liaoning Institute of Transfusion Medicine, Liaoning Blood Center, Shenyang 110044, Liaoning Province, China -
Supported by:Weigao Scientific Research Foundation of Chinese Blood Transfusion Association, No. CSBT-MWG-2021-01 (to LJP); Natural Science Foundation of Liaoning Province, No. 2020-MS-354 (to LXF); Research Project of Shenyang Health Commission, No. 2022096 (to LJ)
CLC Number:
Cite this article
Li Jiao, Li Xiaofeng, Li Jianping. Isolation technique and application of platelet-derived extracellular vesicles from platelet-rich plasma[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 156-163.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
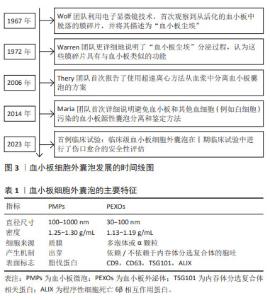
2.1 血小板细胞外囊泡简介 血小板来源于骨髓中的巨核细胞,是血液中没有细胞核结构细胞中最小的血液细胞,保存期较短,一般为5-7 d,但RNA代谢活动非常活跃,不仅具有止血,还具有促进组织再生与修复、免疫等功能。血小板的相关作用依赖于血小板细胞外囊泡,在血浆众多的细胞外囊泡中,约有25%是血小板细胞外囊泡,由活化的血小板或骨髓中的血小板前体细胞巨核细胞释放[5-7], 通过P-选择素和溶酶体相关膜糖蛋白1等活化标记物,可以区分活化血小板释放的细胞外囊泡与巨核细胞产生的细胞外囊泡[8]。 最早有研究通过微分离心法从血小板中分离出颗粒物,后来通过凝血酶生成试验确定了其血小板样活性,但尚未与血小板细胞外囊泡相联系,直到电子显微成像技术的发展,人们才逐渐了解血小板囊泡,近年来,血小板细胞外囊泡不仅因为良好的促凝活性,还因为其促进组织修复和再生的巨大潜力越来越受到关注,见图3。血小板囊泡的产生依赖于血小板的活化,活化的血小板释放的血小板细胞外囊泡主要包括2种类型:血小板微囊泡(platelet-derived microparticles,PMPs)和血小板外泌体(platelet-derived exosomes,PEXOs),见表1。"
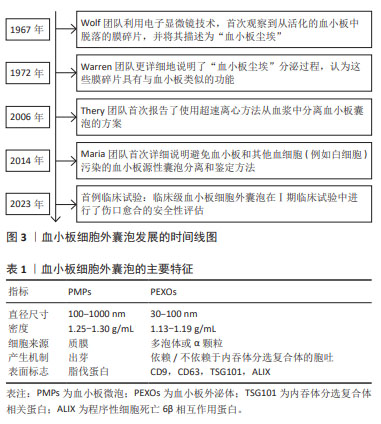
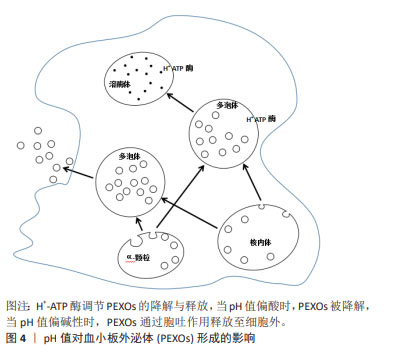
血小板微囊泡是在应激条件下从细胞质膜通过出芽方式释放的囊泡(100-1 000 nm),具有典型的血小板和巨核细胞免疫表型[9]。而具有亚微米直径(30-100 nm)的血小板外泌体起源于多泡体或α-颗粒,通过胞吐途径释放,受环境条件的高度调节,血小板外泌体的组成更为多变,更能反映分泌细胞的生理状态。尽管血小板微囊泡和血小板外泌体在产生途径、直径尺寸及生物学特征方面均存在一定差异,但血小板细胞外囊泡具有一定的重叠性,目前的分离方法和表征技术常无法区分两者,一些研究报告,两者的蛋白质组成可能存在一定的差异,例如血小板微囊泡表面标志物为脂伐蛋白[10],常暴露磷脂酰丝氨酸,而在血小板外泌体中发现了外泌体标记蛋白的高表达,如CD9和CD63等[7,11-13]。 2.2 血小板细胞外囊泡的分泌机制 血小板外泌体不仅可以来源于核内体,还可由α-颗粒产生,这一过程常依赖多泡体中H+-ATP酶的调节[14],见图4。多囊泡体中胆固醇的积累会激活H+-ATP酶,促进血小板外泌体的降解,相反中性鞘磷脂酶2抑制H+-ATP酶活性,导致血小板外泌体的分泌增加[15],一旦多泡体形成,多泡体膜将与质膜融合,内吞体分选转运复合体在多泡体的形成及血小板外泌体的分泌过程中起到了重要作用,是血小板外泌体形成的最重要机制[16-19],然而也有研究表明,外泌体也可以通过非内吞体分选转运复合体机制分泌[20]。 "
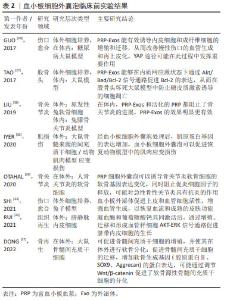
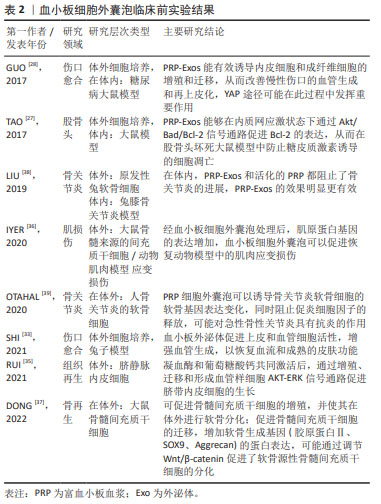
血小板微囊泡形成的相关机制目前还不清楚,但有证据表明,肌动蛋白细胞骨架在血小板微囊泡的形成过程具有重要作用。肌动蛋白可能通过3个步骤调控血小板微囊泡形成的过程:首先,肌动蛋白聚合产生的力可以导致质膜中芽的形成[21];其次,细胞中的肌球蛋白酶可以将这些成分运输到血小板微囊泡产生的位置;最后,肌动蛋白肌球蛋白网络可以闭合“芽体”并与宿主细胞分离。肌动蛋白聚合过程中细胞骨架的不稳定性或发生的结构重排,导致血小板微囊泡释放量增加[14,22]。此外,血小板微囊泡的形成还与血小板外膜上的磷脂酰丝氨酸暴露密切相关,磷脂酰丝氨酸残基在外膜上表达,细胞膜上磷脂酰丝氨酸不对称性的丧失与血小板微囊泡形成相一致,在有磷脂酰丝氨酸暴露缺陷的患者中,发现血小板细胞外囊泡水平降低,阐明了磷脂酰丝氨酸在血小板微囊泡形成中的重要性[23-24]。 2.3 血小板细胞外囊泡在再生医学的应用 2.3.1 血小板细胞外囊泡的应用领域 近几十年,富血小板血浆在再生医学领域的应用逐渐增多,应用领域包括皮肤创面修复、肌肉骨骼再生、心血管疾病、神经再生、美容及疼痛治疗等。富血小板血浆主要通过释放的多种生物活性分子介导组织再生过程,主要因子包括:①初级生长因子:包括血小板源性生长因子、转化生长因子、胰岛素样生长因子、血管内皮生长因子、表皮生长因子、成纤维细胞生长因子及肝细胞生长因子等;②黏附分子:包括纤维蛋白原、黏附蛋白、纤维连接蛋白、玻璃体连接蛋白和血栓形成反应蛋白1和趋化因子等。 尽管富血小板血浆的治疗很有前途,但临床应用仍然受到一些限制。首先,许多富血小板血浆血浆衍生的生物分子一旦从血小板中释放出来,就无法受到磷脂膜的保护,可能被细胞外环境的溶解酶破坏,并迅速失去其生物活性。其次,由于富血小板血浆的制备和临床应用缺乏统一的标准,而且富血小板血浆的成分常具有一定的异质性,受捐献者个体的特征影响,包括性别、年龄和健康状况等导致富血小板血浆的治疗结果难以比较,并可能导致许多研究无法重复验证。基于以上研究,近几年,血小板细胞外囊泡引起了研究者的关注,陆续开展了血小板细胞外囊泡的应用研究,目前已在在组织损伤、肌肉再生、血管生成、骨再生和骨关节炎等再生医学领域已有临床前报道[4]。最近,基于配体的外泌体亲和纯化色谱方案制备的临床级血小板细胞外囊泡在Ⅰ期临床试验中进行了伤口愈合的安全性评估,没有明显的不良事件报道,这表明血小板细胞外囊泡可以安全地用于人类,并值得进一步临床试验验证对伤口愈合疗效,为临床级血小板细胞外囊泡对人类的安全性和治疗效用提供了第一个证据[25]。 目前,血小板细胞外囊泡的应用研究主要包括两方面。一方面在体外,从细胞增殖、分化、迁移等方面研究血小板细胞外囊泡对鼠、兔和猪等动物细胞和人源细胞行为的影响,细胞类型包括间充质干细胞、成纤维细胞、内皮细胞、软骨细胞及神经细胞等。体外研究发现,活化的血小板细胞外囊泡可以促进人脐静脉内皮细胞的增殖和迁移,以及miR-126和血管生成因子的高表达[26]。体内研究也证实了血小板细胞外囊泡对血管生成和缺血后血管重建的重要作用,血小板细胞外囊泡通过诱导局部血管生成促进股骨头坏死后骨组织的修复和再生[27],也有研究认为血小板细胞外囊泡促进慢性皮肤创面再上皮化的重要机制之一是促进局部血管生成,而且,血小板细胞外囊泡具有剂量依赖性的血管生成效应[28]。血小板细胞外囊泡对多种类型细胞有积极影响的证据越来越多。血小板细胞外囊泡治疗可以显著增强血管内皮细胞的生物学行为,包括招募、增殖、迁移和血管形成能力,并促进局部血管生成;血小板细胞外囊泡可以通过YAP去磷酸化,显著增加原代皮肤成纤维细胞的增殖和迁移,增加结缔组织生长因子的分泌,加速创面愈合[28];血小板细胞外囊泡可有效抑制小鼠成骨细胞和间充质干细胞的细胞凋亡,促进其增殖和成骨分化[27];在神经干细胞方面,血小板细胞外囊泡也被认为可以增强细胞的增殖和存活,并增加向神经胶质细胞和神经元分化的潜力[29]。 众所周知,血小板具有促凝止血特性,然而研究表明,血小板细胞外囊泡表面的促凝剂是活化血小板的50-100倍[3]。一方面,血小板细胞外囊泡中含有许多凝血相关物质,显著提高了血小板细胞外囊泡的促凝功能;另一方面,血小板细胞外囊泡膜表面带有负电荷的磷脂酰丝氨酸暴露,促进凝血酶生成,在止血中发挥重要作用[30]。在体外研究发现,血小板细胞外囊泡可以促进止血,改善创伤后出血,含有促凝表型的血小板细胞外囊泡能够促进血栓的形成[31-32]。 另一方面,通过建立鼠和兔等动物模型在体外研究血小板细胞外囊泡的再生医学相关作用。美国梅奥诊所的研究组在难治性慢性伤口的临床前研究中,通过记录家兔模型皮肤完整性、毛囊、汗腺、皮肤油脂和正常水合作用的恢复情况,发现纯化的血小板细胞外囊泡可以促进伤口愈合、皮肤再生,达到无瘢痕的皮肤愈合状态,而且血小板细胞外囊泡可制成干粉,在室温条件长期保存,在手术室或床旁即可将粉末与水凝胶溶液混合后直接涂在伤口上,使用方便[33]。研究发现,当组织中存在炎症时,血小板细胞外囊泡可以通过促进炎症细胞相互作用,促进组织中炎症细胞浸润,破坏内皮细胞,从而加重组织损伤。当无炎症或抗炎因子大于促炎因子时,血小板细胞外囊泡可通过促进组织再生、成纤维细胞增殖和迁移、内皮细胞增殖等方式促进组织修复[34]。 总之,血小板细胞外囊泡确实具有诸多超越富血小板血浆的优势条件[28-39],例如:①生产因子浓度更高尺寸较小;②可通过生物屏障;③免疫原性更低;④具有磷脂双层分子的保护,更稳定内容物丰富,有助于细胞间通讯;⑤半衰期更长,保存更方便等,随着大量的临床应用研究报告,血小板细胞外囊泡有望取代富血小板血浆,成为组织修复和再生领域更有效和更安全的临床候选产品。 文章列举了血小板细胞外囊泡临床前实验成果,见表2。"
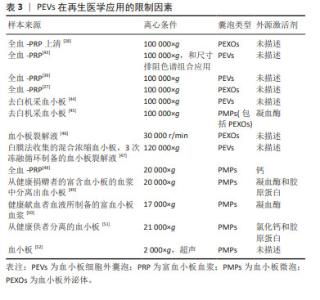
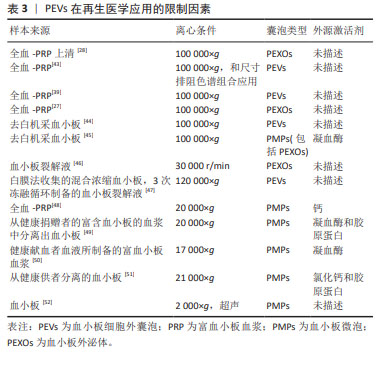
2.3.2 血小板细胞外囊泡在再生医学应用的限制因素 分离方法:2006年,研究者首次报告了使用超速离心方法从血浆中分离血小板细胞外囊泡的方案[40],其原理为利用不同转速的离心力,逐步去除细胞、细胞碎片及大分子干扰物,通常先低速离心保留上清液中小于1 μm的颗粒,随后进行差速离心将较小和密度较低的组分与较大或较重的组分分离。根据血小板微囊泡和血小板外泌体的大小和密度的差异,多数研究者认为通过低速离心 (21 000×g以下)可分离血小板微囊泡,高速离心(100 000×g以上)可分离血小板外泌体或与血小板微囊泡的混合物。然而,由于尺寸的重叠,通过超速离心方法从血小板微囊泡和凋亡小体中分离出纯的血小板外泌体也是不可能的。由于超速离心法易受血浆蛋白的干扰,大多数研究多选择超速离心法或与其他方法联合分离血小板细胞外囊泡,其中最常用的方法为尺寸排除色谱,尺寸排除色谱是以不同粒径的聚合物凝胶作为分离介质填充在分离柱中,当样本进入时,样品分子按其分子大小先后排阻,从凝胶柱中流出,因此可达到不同粒径分子的分离效果。 已有研究表明,尺寸排除色谱去除了囊泡中99%的可溶性血浆蛋白和> 95%的高密度脂蛋白,而不诱导囊泡聚集同时保持了它们的完整性和生物活性[41],尺寸排除色谱和超速离心法两者结合,可以弥补自身的缺点获得比较纯净的血小板细胞外囊泡。尽管还有一些研究根据免疫表型特异性,选择单克隆抗体和磁珠免疫捕获技术分离血小板细胞外囊泡亚群,但由于抗体的交叉反应性、血浆中的其他配体的干扰以及洗脱困难等缺陷,同时由于血小板细胞外囊泡的异质性,也存在分离偏差。总之,在缺乏所有血小板细胞外囊泡均表达的共同标记的情况下,不可能分离出全部血小板细胞外囊泡群体,在缺乏能够唯一性识别血小板细胞外囊泡亚型的标记物的条件下,也很难区分血小板微囊泡和血小板外泌体。研究发现,用尺寸排除色谱从血浆中分离血小板细胞外囊泡,然后通过双离心浓缩,获得的血小板细胞外囊泡纯度和浓度更高[42],但尺寸排除色谱常会分离所有血浆囊泡,因此分离血小板细胞外囊泡的样本来源及类型也会对血小板细胞外囊泡分离技术产生一定的影响。 文章总结了血小板细胞外囊泡在再生医学应用中的限制因素[27-28,39,43-52],见表3。"
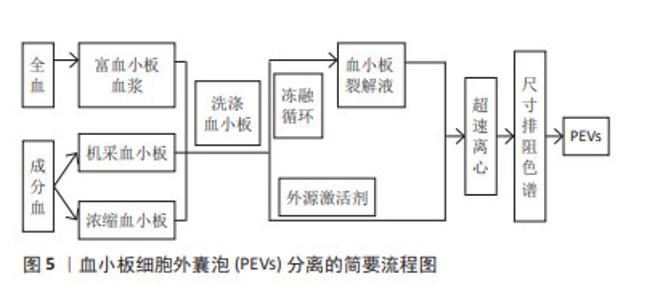
样本来源:根据分离血小板细胞外囊泡样本的血液成分差异,新鲜全血和血小板浓缩物(浓缩血小板、机采血小板等)均可用于分离血小板细胞外囊泡,见表3,图5。但目前的技术条件还无法从混合囊泡中精准鉴别出不同种类的囊泡,从降低红细胞、白细胞等其他血细胞囊泡的污染角度考虑,血小板浓缩物较新鲜全血更适合分离血小板细胞外囊泡。若选择全血,抗凝全血较不抗凝血更具有优势,血清在体外凝块形成过程中血小板衍生颗粒水平的明显升高,从而导致样本不能完全代表原始囊泡组成,干扰下游研究分析[53],抗凝全血需先分离出富血小板血浆成分,再进行血小板细胞外囊泡分离。由于血小板在样本操作过程中很容易被激活并分泌血小板细胞外囊泡,因此,血小板细胞外囊泡分离方案的样本来源应该把防止血小板活化作为考虑因素之一,为降低体外血小板活化以及导致的血小板细胞外囊泡释放,国际血栓形成和止血学会推荐将柠檬酸钠作为全血抗凝剂[54]。根据样本是否活化,血小板细胞外囊泡可以来源于非外源活化的血小板裂解液,也可来源于活化的富血小板血浆及其上清液[27-28,35,38,55]。前者是血小板的裂解纯化物,是一种无细胞上清液,可通过超声、冻融等方法制备。目前血小板细胞外囊泡研究的样本来源多是添加外源激活剂活化后的富血小板血浆,以刺激血小板细胞外囊泡大量释放,但激活剂对血小板细胞外囊泡的形成机制、分子组成,甚至生理功能都具有重要的影响作用。 "
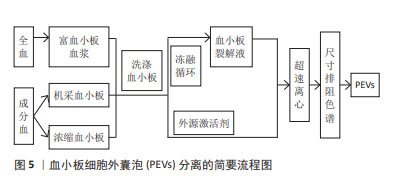
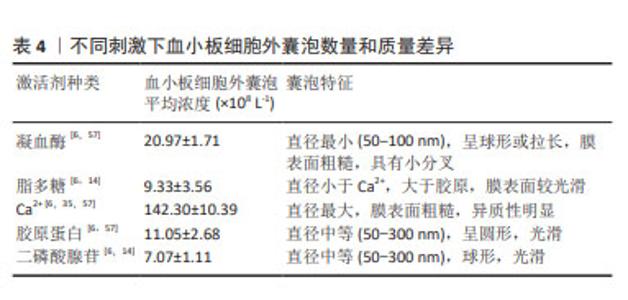
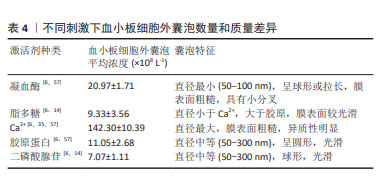
激活剂:物质分泌是血小板活化的重要结果,血小板细胞外囊泡与其他血细胞囊泡分离的主要不同之处常是需要先激活后分离,血小板细胞外囊泡的释放依赖于血小板的活化,激活剂决定血小板细胞外囊泡的生物学特征,不仅仅是其分子组成,甚至影响血小板细胞外囊泡的生物学功能。目前研究较多的激活剂包括凝血酶、Ca2+、胶原蛋白以及二磷酸腺苷等[56]。已有研究证明,不同血小板激活剂对血小板细胞外囊泡亚群的数量和质量有不同的影响。研究发现,与单独使用凝血酶或葡萄糖酸钙刺激相比,凝血酶和葡萄糖酸钙一起刺激,产生的血小板外泌体浓度和细胞因子更高[35]。在一项不同刺激剂对血小板细胞外囊泡释放量影响的研究中发现,用Ca2+刺激血小板产生最高浓度的血小板细胞外囊泡,凝血酶刺激组血小板细胞外囊泡释放量次之,二磷酸腺苷刺激组血小板细胞外囊泡释放量最低[6],虽然目前没有文章详细说明如何选择激活血小板产生血小板细胞外囊泡的激动剂,但由于刺激条件对血小板细胞外囊泡的生物组成,甚至是生物学功能具有重要的影响,由静止的血小板和受激动剂刺激的血小板产生的血小板细胞外囊泡的结构多样性和尺寸是不同的[57],应该从研究和应用的目的,选择合适的激活剂激活血小板。具体见表4。 "
| [1] VAJEN T, MAUSE SF, KOENEN RR. Microvesicles from platelets: novel drivers of vascular inflammation. Thromb Haemost. 2015;114(2): 228-236. [2] WU J, PIAO Y, LIU Q, et al. Platelet-rich plasma-derived extracellular vesicles: a superior alternative in regenerative medicine? Cell Proliferation. 2021;54(12):e13123. [3] SINAURIDZE EI, KIREEV DA, POPENKO NY, et al. Platelet microparticle membranes have 50-to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97(3):425-434. [4] ROSSELLO MA, GENESTRA MAF, MONJO M, et al. Platelet-derived extracellular vesicles for regenerative medicine. Preprints. 2021; 22(16):8580. [5] ARRAUD N, LINARES R, TAN S, et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12(5):614-627. [6] AATONEN MT, OHMAN T, NYMAN TA, et al. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles. 2014;6:3. [7] SPAKOVA T, JANOCKOVA J, ROSOCHA J. Characterization and therapeutic use of extracellular vesicles derived from platelets. Int J Mol Sci. 2021; 22(18):9701. [8] FLAUMENHAFT R, DILKS JR, RICHARDSON J, et al. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;5:113. [9] ITALIANO JE, MAIRUHU AT, FLAUMENHAFT R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17(6):578-584. [10] GANGALUM RK, ATANASOV IC, ZHOU ZH, et al. αB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J Biol Chem. 2011;286(5):3261-3269. [11] MOREL O, JESEL L, FREYSSINET JM, et al. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31(1):15-26. [12] VEDPATHAK S, SHARMA A, PALKAR S, et al. Platelet derived exosomes disrupt endothelial cell monolayer integrity and enhance vascular inflammation in dengue patients. Front Immunol. 2024;14:1285162. [13] TRIPISCIANO C, RENE W, EICHHORN T, et al. Different potential of extracellular vesicles to support thrombin generation: contributions of phosphatidylserine, tissue factor, and cellular origin. Sci Rep. 2017; 7(1):6522. [14] EUSTES AS, DAYAL S. The role of platelet-derived extracellular vesicles in immune-mediated thrombosis. Int J Mol Sci. 2022;23(14):7837. [15] CHOEZOM D, GROSS JC. Neutral sphingomyelinase 2 controls exosome secretion by counteracting V-ATPase-mediated endosome acidification. J Cell Sci. 2022;135(5):jcs259324. [16] COLOMBO M, MOITA C, VAN NG, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24): 5553-5565. [17] STOORVOGEL W. Resolving sorting mechanisms into exosomes. Cell Res. 2015;25(5):531-532. [18] COLOMBO M, RAPOSO G, THERY C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-289. [19] TRAJKOVIC K, HSU C, CHIANTIA S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008; 319(5867):1244-1247. [20] WEI DH, ZHAN WX, GAO Y, et al. RAB31 marks and controls an ESCRT-independent exosome pathway.Cell Res. 2021;31(2):157-177. [21] HOLLIDAY LS, FARIA LP, RODY WJ Jr. Actin and actin-associated proteins in extracellular vesicles shed by osteoclasts. Int J Mol Sci. 2019;21(1):158. [22] COLLIER ME, MARAVEYAS A, ETTELAIE C. Filamin-A is required for the incorporation of tissue factor into cell-derived microvesicles. Thromb Haemost. 2014;111(4):647-655. [23] MOREL O, JESEL L, FREYSSINET JM, et al. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31(1): 15-26. [24] LI X, WANG Q. Platelet-derived microparticles and autoimmune Diseases. Int J Mol Sci. 2023;24(12):10275. [25] JOHNSON J, LAW SQK, SHOJAEE M, et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J Extracell Vesicles. 2023;12(7):e12332. [26] SUN Y, LIU XL, ZHANG D, et al. Platelet-derived exosomes affect the proliferation and migration of human umbilical vein endothelial cells via miR-126. Curr Vasc Pharmacol. 2019;17(4):379-387. [27] TAO SC, YUAN T, RUI BY, et al. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7(3):733. [28] GUO SC, TAO SC, YIN WJ, et al. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7(1): 81-96. [29] HAYON Y, DASHEVSKY O, SHAI E, et al. Platelet microparticles induce angiogenesis and neurogenesis after cerebral ischemia. Curr Neurovasc Res. 2012;9(3):185-192. [30] WANG Y, ZHANG S, LUO L, et al. Platelet-derived microparticles regulates thrombin generation via phophatidylserine in abdominal sepsis. J Cell Physiol. 2018;233(2):1051-1060. [31] LOPEZ L, SRIVASTAVA AK, BURCHFIELD J, et al. Platelet-derived- extracellular vesicles promote hemostasis and prevent the developmentof hemorrhagic shock. Sci Rep. 2019;9(1):1-10. [32] DTER MR, ALEXANDER W, HASSOUNE A, et al. Platelet‐derived extracellular vesicles released after trauma promote hemostasis and contribute to DVT in mice. J Thromb Haemost. 2019;17(10):1733-1745. [33] SHI A, LI J, QIU X, et al. TGF-β loaded exosome enhances ischemic wound healing in vitro and in vivo. Theranostics. 2021;11(13):6616-6631. [34] ZHU Z, SUN S, JIANG T, et al. A double-edged sword of platelet-derived extracellular vesicles in tissues, injury or repair: thecurrent research overview. Tissue Cell. 2023;82:102066. [35] RUI S, YUAN Y, DU C, et al. Comparison and investigation of exosomes derived from platelet-rich plasma activated by different agonists. Cell Transpl. 2021;30:9636897211017833. [36] IYER SR, SCHEIBER AL, YAROWSKY P, et al. Exosomes isolated from platelet-rich plasma and mesenchymal stem cells promote recovery of function after muscle injury. Am J Sports Med. 2020;48:2277-2286. [37] DONG B, LIU X, LI J, et al. Berberine encapsulated in exosomes derived from platelet-rich plasma promotes chondrogenic differentiation of the bone marrow mesenchymal stem cells via the Wnt/β-catenin pathway. Biol Pharm Bull. 2022;45:1444-1451. [38] LIU X, WANG L, MA C, et al. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J Orthop Surg Res. 2019;14(1):470. [39] OTAHAL A, KRAMER K, KUTEN PO, et al. Characterization and chondroprotective effects of extracellular vesicles from plasma- and serum-based autologous blood-derived products for osteoarthritis therapy. Front Bioeng Biotechnol. 2020;8:584050. [40] THÉRY C, AMIGORENA S, RAPOSO G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30(1):3-22. [41] TAMAS B, HERCZEG K, ZSOFIA O, et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. 2015;10(12):e0145686. [42] FERREIRA PM, BOZBAS E, TANNETTA SD, et al. Mode of induction of platelet-derived extracellular vesicles is a critical determinant of their phenotype and function. Sci Rep. 2020;10(1):18061. [43] OTAHAL A, KUTEN PO, KRAMER K, et al. Functional repertoire of EV-associated miRNA profiles after lipoprotein depletion via ultracentrifugation and size exclusion chromatography from autologous blood products. Sci Rep. 2021;11(1):5823. [44] MIYAZAWA B, TRIVEDI A, TOGARRATI PP, et al. Regulation of endothelial cell permeability by platelet-derived extracellular vesicles. J Trauma Acute Care Surg. 2019;86(6):931-942. [45] AYON Y, DASHEVSKY O, SHAI E, et al. Platelet microparticles promote neural stem cell proliferation, survival and differentiation. J Mol Neurosci. 2012;47(3):659-665. [46] TORREGGIANI E, PERUT F, RONCUZZI L, et al. Exosomes:novel effectors of human platelet lysate activity. Eur Cell Mater. 2014;28: 137-151. [47] ANTICH RM, FORTEZA GMA, CALVO J, et al. Platelet-derived extracellular vesicles promote osteoinduction of mesenchymal stromal cells. Bone Joint Res. 2020;9(10):667-674. [48] LOVISOLO F, CARTON F, GINO S, et al. Platelet rich plasma-derived microvesicles increased in vitro wound healing. Eur Rev Med Pharmacol Sci. 2020;24(18):9658-9664. [49] MAUSE SF, RITZEL E, LIEHN EA, et al. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122(5):495. [50] KIM HK, SONG KS, JUN C, et al. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2015;124(3)376-384. [51] LIANG C, HUANG J, LUO P, et al. Platelet-derived microparticles mediate the intra-articular homing of mesenchymal stem cells in early-stage cartilage lesions. Stem Cells Dev. 2020;29(7):414-424. [52] MOEST T, KOEHLER F, PRECHTL C, et al. Bone formation in peri-implant defects grafted with microparticles: a pilot animal experimental study. J Clin Periodontol. 2014;41(10):990-998. [53] ZHANG X, TAKRUCHI T, TAKEDA A, et al. Comparison of serum and plasma as a source of blood extracellular vesicles: increased levels of platelet-derived particles in serum extracellular vesicle fractions alter content profiles from plasma extracellular vesicle fractions. PLoS One. 2022;17(6):e0270634. [54] LACROIX R, JUDICONE C, MOOBERR M, et al. Standardization of pre-analytical variables in plasmamicroparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2013;11:1190-1193. [55] SAUMELL-ESNAOLA M, DELGADO D, GARCIA DCG, et al. Isolation of platelet-derived exosomes from human platelet-rich plasma: biochemical and morphological characterization. Int J Mol Sci. 2022; 23(5):2861. [56] GARDIN C, FERRONI L, LEO S, et al. Platelet-derived exosomes in atherosclerosis. Int J Mol Sci. 2022;23(20):12546. [57] PONOMAREVA AA, NEVZOROVA TA, MORDAKHANOVA ER, et al. Intracellular origin and ultrastructure of platelet-derived microparticles. J Thromb Haemost. 2017;15(8):1655-1667. [58] ANTICH RM, FORTEZA GMA, MONJO M, et al. Platelet-derived extracellular vesicles for regenerative medicine. Int J Mol Sci. 2021; 22(16):8580. [59] BOUDREAU LH, CUCHEZ AC, CLOUTIER N, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124(14): 2173-2183. [60] JOHNSON J, WU YW, BLYTHl C, et al. Prospective therapeutic applications of platelet extracellular vesicles. Trends Biotechnol. 2020; 39(6):598-612. [61] OWENS AP, MACKMAN N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108(10):1284-1297. [62] CLARK SR, THOMAS CP, HAMMOND VJ, et al. Characterization of platelet aminophospholipid externalization reveals fatty acids as molecular determinants that regulate coagulation. Proc Natl Acad Sci U S A. 2013;110(15):5875-5880. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Han Haihui, Ran Lei, Meng Xiaohui, Xin Pengfei, Xiang Zheng, Bian Yanqin, Shi Qi, Xiao Lianbo. Targeting fibroblast growth factor receptor 1 signaling to improve bone destruction in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1905-1912. |
| [3] | Dong Tingting, Chen Tianxin, Li Yan, Zhang Sheng, Zhang Lei. Causal relationship between modifiable factors and joint sports injuries [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1953-1962. |
| [4] | Deng Keqi, Li Guangdi, Goswami Ashutosh, Liu Xingyu, He Xiaoyong. Screening and validation of Hub genes for iron overload in osteoarthritis based on bioinformatics [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1972-1980. |
| [5] | Zhou Jinhai, Li Jiangwei, Wang Xuquan, Zhuang Ying, Zhao Ying, Yang Yuyong, Wang Jiajia, Yang Yang, Zhou Shilian. Three-dimensional finite element analysis of anterior femoral notching during total knee arthroplasty at different bone strengths [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1775-1782. |
| [6] | Liu Yan, Wang Kai, Wu Min. Relationship between coronal angle fluctuation of ankle point and recovery of joint function after ankle fracture [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1820-1826. |
| [7] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| [8] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [9] | Li Jun, Gong Jingjing, Sun Guobin, Guo Rui, Ding Yang, Qiang Lijuan, Zhang Xiaoli, Fang Zhanhai . miR-27a-3p promotes the proliferation of human hypertrophic scar fibroblasts by regulating mitogen-activated protein kinase signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1609-1617. |
| [10] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [11] | Zhang Yuxin, Yu Cong, Zhang Cui, Ding Jianjun, Chen Yan. Differences in postural control ability between older adults with mild cognitive impairment and those with normal cognition under different single-task and dual-task conditions [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1643-1649. |
| [12] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [13] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [14] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [15] | Zhang Zixian, Xu Youliang, Wu Shaokui, Wang Xiangying. Effects of blood flow restriction training combined with resistance training on muscle indicators in college athletes: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1705-1713. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||