Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (7): 1497-1503.doi: 10.12307/2024.742
Previous Articles Next Articles
Role and mechanism of platelet-derived growth factor BB in repair of growth plate injury
Peng Hongcheng1, 2, Peng Guoxuan1, Lei Anyi1, 2, Lin Yuan1, 2, Sun Hong1, Ning Xu1, Shang Xianwen1, Deng Jin3, Huang Mingzhi1
- 1Department of Orthopedics, 3Department of Emergency Medicine, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 2Clinical Medicine School of Guizhou Medical University, Guiyang 550004, Guizhou Province, China
-
Received:2023-09-06Accepted:2023-12-15Online:2025-03-08Published:2024-06-28 -
Contact:Huang Mingzhi, Master’s supervisor, Chief physician, Department of Orthopedics, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Peng Hongcheng, Master candidate, Department of Orthopedics, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; Clinical Medicine School of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 82360420 (to PGX); Doctoral Research Initiation Fund of Affiliated Hospital of Guizhou Medical University, No. gyfybskj-2023-07 (to PGX); Science and Technology Fund of Guizhou Provincial Department of Health, No. GZWJKJXM20240939 (to PGX); Guizhou Provincial Science and Technology Program Project, No. qkhjc-ZK[2023] General 387 (to HMZ)
CLC Number:
Cite this article
Peng Hongcheng, Peng Guoxuan, Lei Anyi, Lin Yuan, Sun Hong, Ning Xu, Shang Xianwen, Deng Jin, Huang Mingzhi . Role and mechanism of platelet-derived growth factor BB in repair of growth plate injury[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1497-1503.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

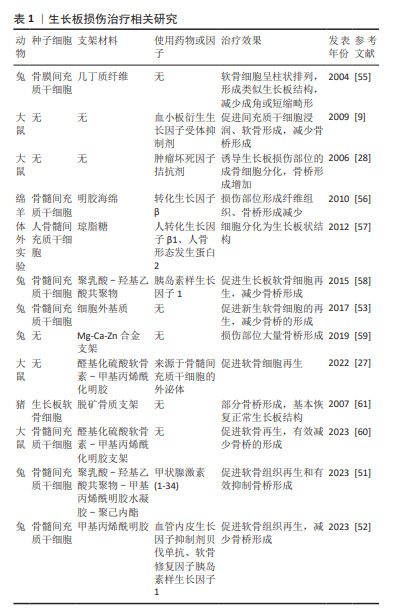
2.1 PDGF-BB功能概述 PDGF在胚胎发育、细胞分化及对组织损伤反应等过程中具有十分重要的作用[11]。已证实PDGF存在血浆中,也可从软骨细胞、成骨细胞、破骨细胞、巨噬细胞、上皮细胞、血管内皮细胞、成纤维细胞、成肌细胞、平滑肌细胞、神经元、间充质干细胞中产生,这决定PDGF可发挥促进器官发生、骨骼发育、血管生成、伤口愈合细胞免疫调控等多种作用[12]。PDGF家族主要由PDGF-A、PDGF-B、PDGF-C和 PDGF-D组成,其产物以二硫键连接成同二聚体或异二聚体的形式分泌:PDGF-AA、PDGF-BB、PDGF-CC、 PDGF-DD和PDGF-AB[13]。PDGF-BB作为其中活性最高、功能最强细胞因子具有促有丝分裂、分化、趋化和血管生成等作用,参与多种疾病的病理过程[14]。研究已证实,PDGF-BB可介导间充质干细胞归巢修复心脏缺血再灌注损伤小鼠心肌细胞发挥心脏保护作用[15]。PDGF-BB也可通过促进血管内皮生长因子分泌而诱导血管生成,促进血管周围细胞的增殖和募集血管内皮细胞[16]。相反,抑制PDGF受体则可有效抑制新生血管的形成,并促进新生血管的凋亡,表明PDGF-BB在维持新血管稳定性方面起着重要作用[17]。在骨骼重建过程中,PDGF-BB可作为干细胞趋化因子促进骨髓间充质干细胞的成骨分化,可有效减缓骨质疏松的骨量丢失[18]。同时,破骨细胞前体来源的PDGF-BB也可通过H型血管参与骨性关节炎、骨质疏松等的病理进程[19]。上述研究结果说明,PDGF-BB在诱导干细胞归巢及增殖分化、维持血管形成等方面发挥了重要作用。在生长板损伤过程中,损伤炎症初期PDGF-BB通过促进间充质祖细胞浸润、软骨形成、成骨反应以及调控骨重塑来促进生长板损伤修复[9]。 生长板损伤经历早期炎症、血管重建、纤维骨化、结构重塑等病理进程,伴随着软骨细胞、血管内皮细胞、干细胞、成骨细胞、破骨细胞多种细胞交叉对话,PDGF-BB可能作为轴心调控参与多种细胞生物学功能,介导生长板损伤的修复。因此,探寻PDGF-BB在生长板损伤过程中的可能作用机制,可能为生长板损伤寻找新的治疗思路。 2.2 PDGF-BB介导炎症反应参与生长板损伤修复 目前,生长板损伤位置的骨重塑是骨桥形成的重要原因,也是后期肢体骨骼发生成角短缩畸形的主要原因,因此,抑制损伤后骨桥形成、促进局部生长板软骨细胞增殖、促进干细胞成软骨分化是修复生长板的重要方法。生长板损伤早期血管扩张、血浆渗出、水肿及炎性细胞(包括嗜中性粒细胞、肥大细胞和巨噬细胞)浸润,刺激生长板软骨细胞、成骨细胞、间充质干细胞等分化并伴有破骨细胞开始清除死骨[4,20]。损伤后来自骨外膜、髓腔及周围软组织增生的纤维组织分隔血肿,吞噬细胞和巨细胞吸收血肿后释放包括PDGF-BB、肿瘤坏死因子α、白细胞介素1β和骨形态发生蛋白等多种信号因子[21]。内源性PDGF-BB的释放可通过与PDGF受体αβ结合激活下游核因子κB和白细胞介素6通路,而白细胞介素6可作为促炎性细胞因子促进炎症发生,并且成骨细胞分泌的白细胞介素6可以刺激破骨细胞形成[22]。也就是说,PDGF-BB可能通过与PDGF受体αβ结合进而激活成骨细胞分泌的白细胞介素6,促进破骨细胞开始清除死骨。因此,如能进一步阐明PDGF-BB介导PDGF受体αβ途径参与成骨细胞白细胞介素6调控破骨细胞的具体分子机制,将为PDGF-BB修复生长板损伤提供重要思路。再者,PDGF-BB的释放通过细胞外调节蛋白激酶信号通路促进骨髓间充质干细胞的成骨细胞分化并抑制其成脂分化,磷脂酰肌醇-3激酶/蛋白激酶B和细胞外调节蛋白激酶信号通路增强了人脐静脉内皮细胞的迁移和血管生成,促进成骨细胞的形成[23]。所以,PDGF-BB在生长板损伤炎症阶段通过激活PDGF受体αβ、核因子κB、白细胞介素6信号促进成骨细胞增殖分化、骨髓间充质干细胞成骨分化以及破骨细胞清除死骨,调控生长板损伤后的骨桥重建。而损伤处募集的血小板、巨噬细胞和肥大细胞也能产生PDGF-BB,损伤处血小板可分泌PDGF-BB促进中性粒细胞和巨噬细胞浸润,促进成纤维细胞分泌新的细胞外基质,这可能促进了损伤炎症期向成纤维期过渡。 PDGF-BB由生长板损伤后早期损伤区域中性粒细胞释放的白细胞介素6、趋化因子受体2招募单核巨噬细胞分泌,巨噬细胞也可分泌肿瘤坏死因子α、白细胞介素6、白细胞介素1等细胞因子刺激破骨细胞参与生长板损伤后骨重建[24]。与此同时,生长板损伤后生长板软骨细胞的矿化也受到炎症调控,炎症对软骨基质蛋白的抑制作用导致细胞外基质逐渐丢失,极大地阻碍生长板软骨细胞的再生并导致生长板软骨细胞矿化,最终导致生长板骨桥形成[20,25]。既往研究证实,生长板损伤早期阶段PDGF-BB基因的表达显著上调,且PDGF-BB定位于部分炎症细胞(包括中性粒细胞、巨噬细胞及肥大细胞),而损伤生长板区域存在一定比例PDGF受体-β+间充质基质细胞,这可能是来源于炎症细胞的PDGF-BB诱导PDGF受体-β+间充质基质细胞参与生长板损伤修复,而PDGF受体抑制剂伊马替尼延迟受损生长板软骨和骨组织的形成,导致损伤修复过程延长[9]。胫骨结节骨骺炎(亦称Osgood-Schlatter病)亦是因创伤或劳损引起的胫骨结节骨骺软骨无菌性炎症,可导致骺软骨发生矿化形成异常凸起结节[26]。GUAN等[27]使用外泌体负载的具有抗炎特性的模拟细胞外基质水凝胶促进生长板损伤修复,证实早期抑制生长板损伤后炎症反应能有效修复生长板损伤。ZHOU等[28]在大鼠生长板损伤模型中使用肿瘤坏死因子α拮抗剂,发现阻断肿瘤坏死因子α可通过激活p38丝裂原活化蛋白激酶抑制间充质干细胞的迁移和细胞增殖,同时抑制损伤部位的骨细胞分化和骨基质合成,进而抑制生长板损伤骨桥形成。在生长板损伤部位骨修复过程中,ARASAPAM等[29] 观察到损伤诱导的炎症递质、环氧合酶2和一氧化氮合酶在促进间充质细胞向软骨细胞分化和促进骨重塑中发挥作用。而生长板损伤部位炎性细胞、中性粒细胞的消耗或肿瘤坏死因子α受体活性抑制可增加生长板损伤部位骨组织形成,参与调控软骨内成骨[30]。因此,PDGF-BB作为损伤早期炎症反应的重要因子通过介导多种细胞炎症反应调控损伤修复过程,靶向PDGF-BB介导的炎症刺激可能通过改善破骨细胞、成骨细胞、软骨细胞功能活性延缓生长板损伤骨桥形成进程,实现生长板损伤修复。 2.3 PDGF-BB调节血管形成并偶联软骨内成骨发生参与生长板损伤修复 在生长板损伤骨桥形成过程中,新生血管的增生促进膜内化骨和软骨内骨化逐渐将两断端的纤维性骨痂粘连一起[31]。血管生成是生长板损伤骨桥形成的重要原因,血管桥异常形成导致生长板损伤后软骨细胞快速骨化[32]。内皮细胞定向迁移与血管侵入是纤维组织转化为软骨、软骨发生软骨内骨化形成原始骨痂的启动因素[33]。血管生成包括血管内皮细胞的迁移和增殖、毛细血管的形成,多种血管生成生长因子、细胞因子和其他信号分子参与调节血管生成[33]。抑制血管的入侵和形成可能一定程度延缓生长板损伤后的骨桥形成,防止骨桥快速形成以及促进原位生长板软骨细胞增殖是防止生长板损伤畸形的关键措施[34]。软骨内成骨为骨损伤修复的关键程序,损伤处间充质干细胞分化成软骨形成细胞,而原始骨痂中的软骨不断以软骨内骨化的方式生成新骨,实现骨重建修复[35]。因此,能够围绕血管形成开发出新的策略来操纵骨软骨骨化期间控制软骨细胞向成骨细胞转分化的信号,为生长板损伤和疾病提供治疗上的益处。 骨折后骨折端的纤维组织首先转变为软骨组织,然后软骨细胞逐步转变为成骨细胞,软骨组织最终被骨组织所取代[36]。无论是骨骼发育、生长板软骨内骨化亦或是骨折损伤软骨内成骨,内皮细胞均扮演重要角色[37]。KUSUMBE等[38]首次发现位于胫骨干骺端高表达两种内皮细胞标记抗体血小板——内皮细胞黏附分子(CD31)、内皮黏蛋白(Endomucin,EMCN)抗体的特殊亚型血管(CD31hiEMCNhi,简称H亚型血管),能够与干骺端成骨细胞、破骨细胞以及间充质干细胞等交叉对话调节骨内血管生成和骨形成。位于生长板软骨肥大层下方骨与软骨交界处的破骨细胞前体和成骨前体细胞共同侵入软骨组织肥大区,协同H型血管侵入生长板软骨基质,进而调控软骨内骨化过程,H型血管在骨形成和修复过程中起着重要的调节作用。PDGF-BB可以动员间充质来源的细胞、稳定新形成的血管,从而为成骨细胞的分化协调细胞成分[39]。间充质干细胞与内皮细胞的相互作用分泌的PDGF-BB已被认为是创伤愈合和组织修复的主要介质,PDGF-BB通过促进血管内皮生长因子而具有介导血管生成的功能[40]。XIE等[10,40]提出PDGF-BB在骨重塑过程中介导H型血管和骨形成偶联,并且确定了破骨细胞前体分泌的PDGF-BB是骨髓和外周血中PDGF-BB的主要来源。破骨细胞前体分泌的PDGF-BB不仅可以促进内皮祖细胞和间充质干细胞的迁移,还可以促进其分化为血管形成的表型,诱导H型血管形成,从而促进血管生成与骨形成偶联[10,41]。去卵巢骨质疏松小鼠骨髓和外周血中的PDGF-BB浓度显著降低,骨髓中H型血管也随之减少,敲除组织蛋白酶K或注射组织蛋白酶K抑制剂能够显著提高PDGF-BB浓度,进而增加分布在生长板软骨下骨的H型血管密度,可能为生长板软骨内成骨提供营养支持[10,42]。PDGF-BB也可通过与其受体β结合促进骨髓间充质干细胞和骨髓源性内皮祖细胞的迁移和分化,增加骨形成区域H型血管和成骨细胞[43]。与此同时,抑制PDGF-BB可通过下调血管内皮生长因子的表达来减少血管形成与新骨形成偶联,减少或延缓骨桥形成[44]。唾液酸结合免疫球蛋白样凝集素抗体通过提高抗酒石酸酸性磷酸酶阳性单核细胞的释放PDGF-BB来介导H型血管生成,从而防止骨质疏松和促进骨折愈合[45]。GAO等[46]也发现皮质骨上抗酒石酸酸性磷酸酶染色阳性细胞也可以通过分泌PDGF-BB诱导骨髓间充质干细胞向骨膜迁移并表达骨膜蛋白,促进骨膜血管的增加和成骨细胞功能的增强。因此,抑制抗酒石酸酸性磷酸酶染色阳性单核细胞PDGF-BB的产生可能通过抑制骨膜血管生成来减少骨形成。 综上所述,抑制PDGF受体可有效抑制新生血管的形成、促进新生血管的凋亡,这可能阻断生长板软骨内成骨、抑制生长板损伤骨形成,因此抑制PDGF-BB启动的血管形成与软骨内成骨之间的偶联效应,将有可能实现生长板损伤修复。 2.4 组织工程在生长板损伤修复的应用进展 早期炎症反应、血管形成及新骨形成偶联可能是PDGF-BB参与生长板损伤骨桥形成的重要核心,抑制生长板损伤后血管入侵和新骨形成是早期修复生长板损伤的关键[10,16,40,43-44,47]。 软骨组织工程、间充质干细胞等种子细胞的发展与应用,为预防生长板损伤后骨桥形成、软骨再生提供了潜在的干预措施[48]。组织工程技术和干细胞技术被认为是修复生长板损伤最有效的方法。AZARPIPA等[49]使用4周龄兔构建股骨远端生长板损伤模型后分别植入不同浓度的兔骨髓间充质干细胞,发现间充质干细胞组股骨畸形角度小于对照组。CUNNIFFE等[50]使用生长板处的细胞外基质作为支架,发现该支架能够通过膜内或软骨内途径支持间充质干细胞的成骨,植入动物缺损模型后观察到该支架可加速血管生长、矿化和新生骨形成。GUAN等[27]的研究证实,外泌体联合细胞外基质复合水凝胶可通过抑制生长板损伤早期炎症反应(白细胞介素10、肿瘤坏死因子α、一氧化氮合酶)促进生长板损伤的修复。FAN等[51]制备了甲状腺激素(1-34)@聚乳酸-羟基乙酸共聚物/ 骨髓间充质干细胞/甲基丙烯酰明胶-聚己内酯支架,证实该支架可通过促进软骨再生和减少骨桥来预防生长板损伤后肢体畸形的发生。QIANG等[52]制备了外层为血管内皮生长因子抑制剂、内层为软骨修复因子胰岛素样生长因子1的双层载药微球,并将微球与骨髓间充质干细胞在明胶甲基丙烯酰水凝胶中结合,发现该复合水凝胶能有效抑制生长板血管生成并促进软骨再生。LI等[53]将三维结构的软骨组织工程支架和自体骨髓间充质干细胞植入缺损部位,发现既能够填补生长板处的缺损、提供机械支持,同时也为干细胞和活性物质发挥功能提供了物质载体,发挥抑制骨桥形成、促进生长板损伤修复作用。 随着对生长板发育与生长板损伤后病理机制的深入研究,种子细胞、生长因子、组织工程支架三大因素的合理应用将有效修复损伤的生长板[54-61](表1)。种子细胞(如生长板软骨细胞、软骨细胞、间充质干细胞)联合相关细胞因子(如胰岛素样生长因子1、转化生长因子因子β、甲状腺激素相关蛋白、印度刺猬因子、PDGF-BB等)可能促软骨细胞大量再生、抑制血管和骨形成,进而发挥生长板损伤修复作用[62-63]。PDGF-BB作为一种参与血管生成、骨形成以及炎症调控的重要因子,可能在生长板损伤修复中发挥关键作用。抑制表皮生长因子受体活性可抑制软骨内骨化,表皮生长因子受体能够减少生长板肥大软骨细胞中核因子κB受体激活因子配体的表达,而核因子κB受体激活因子配体是参与破骨细胞、破骨前体细胞调节骨形成的重要调控因子[64-66]。破骨前体细胞分泌的PDGF-BB是介导骨形成和H血管形成的重要因子,控制着生长板软骨下骨转化及骨基质形成等关键步骤,提示如能结合组织工程方法抑制生长板肥大软骨细胞核因子κB受体激活因子配体介导的软骨成骨,可能是防止生长板损伤后软骨异常矿化、骨桥形成的重要策略。再者,生长板损伤后H型血管侵入生长板导致骨桥形成,如能利用组织工程技术靶向抑制PDGF-BB或抑制破骨前体细胞PDGF-BB生成,进而减少H型血管生成与骨形成偶联,这可能为生长板损伤后骨桥形成的预防提供新的思路。除此之外,构建细胞载体及缓释工程支架、保证软骨细胞存活再生、维持细胞因子持续释放均可能成为生长板损伤的组织工程修复方法。再者,组织工程支架的机械强度、良好的生物相容性以及仿生性能可能充分发挥PDGF-BB在生长板损伤中的作用,进一步实现抑制血管新生及骨桥耦联,有效促软骨再生。综上所述,随着组织工程技术的不断发展以及生长板损伤发病机制的不断深入,将为后续临床治疗生长板损伤提供更多的治疗手段。"
| [1] KRONENBERG HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332-336. [2] SAMET JD. Pediatric Sports Injuries. Clin Sports Med. 2021;40(4): 781-799. [3] YAMAMURA MK, CARRY PM, GIBLY RF, et al. Epidemiology of Physeal Fractures and Clinically Significant Growth Disturbances Affecting the Distal Tibia, Proximal Tibia, and Distal Femur: A Retrospective Cohort Study. J Am Acad Orthop Surg. 2023;31(11):e507-e515. [4] CHUNG R, FOSTER BK, XIAN CJ. Injury responses and repair mechanisms of the injured growth plate. Front Biosci (Schol Ed). 2011;3(1):117-125. [5] HALLETT SA, ONO W, ONO N. Growth Plate Chondrocytes: Skeletal Development, Growth and Beyond. Int J Mol Sci. 2019;20(23):6009. [6] STEGEN S, VAN GASTEL N, CARMELIET G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone. 2015;70:19-27. [7] ZHANG J, PAN J, JING W. Motivating role of type H vessels in bone regeneration. Cell Prolif. 2020;53(9):e12874. [8] FRIEDLAENDER GE, LIN S, SOLCHAGA LA, et al.The Role of Recombinant Human Platelet-derived Growth Factor-BB (rhPDGF-BB) in Orthopaedic Bone Repair and Regeneration. Curr Pharm Des. 2013;19(19): 3384-3390. [9] CHUNG R, FOSTER BK, ZANNETTINO AC, et al. Potential roles of growth factor PDGF-BB in the bony repair of injured growth plate. Bone. 2009; 44(5):878-885. [10] XIE H, CUI Z, WANG L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20(11):1270-1278. [11] GUÉRIT E, ARTS F, DACHY G, et al. PDGF receptor mutations in human diseases. Cell Mol Life Sci. 2021;78(8):3867-3881. [12] RAUNIYAR K, BOKHARAIE H, JELTSCH M. Expansion and collapse of VEGF diversity in major clades of the animal kingdom. Angiogenesis. 2023;26(3):437-461. [13] KAZLAUSKAS A. PDGFs and their receptors. Gene. 2017;614:1-7. [14] ZAIDI M, LIZNEVA D, YUEN T. The role of PDGF-BB in the bone-vascular relationship during aging. J Clin Invest. 2021;131(20):e153644. [15] XU B, LUO Y, LIU Y, et al. Platelet-derived growth factor-BB enhances MSC-mediated cardioprotection via suppression of miR-320 expression. Am J Physiol Heart Circ Physiol. 2015;308(9):H980-H989. [16] CHUNG R, FOSTER BK, XIAN CJ. The potential role of VEGF-induced vascularisation in the bony repair of injured growth plate cartilage. J Endocrinol. 2014;221(1):63-75. [17] GIANNI-BARRERA R, BUTSCHKAU A, UCCELLI A, et al. PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis. 2018;21(4):883-900. [18] ZHANG M, YU W, NIIBE K, et al. The Effects of Platelet-Derived Growth Factor-BB on Bone Marrow Stromal Cell-Mediated Vascularized Bone Regeneration. Stem Cells Int. 2018;2018:3272098. [19] SU W, LIU G, LIU X, et al. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight. 2020;5(8):e135446. [20] ZHOU FH, FOSTER BK, SANDER G, et al. Expression of proinflammatory cytokines and growth factors at the injured growth plate cartilage in young rats. Bone. 2004;35:1307-1315. [21] DEL ROSARIO C, RODRÍGUEZ-ÉVORA M, REYES R, et al. BMP-2, PDGF-BB, and bone marrow mesenchymal cells in a macroporous β-TCP scaffold for critical-size bone defect repair in rats. Biomed Mater. 2015; 10(4):045008. [22] TAKEUCHI T, YOSHIDA H, TANAKA S. Role of interleukin-6 in bone destruction and bone repair in rheumatoid arthritis. Autoimmun Rev. 2021;20(9):102884. [23] LIU B, LI J, CHEN B, et al. Dental pulp stem cells induce anti-inflammatory phenotypic transformation of macrophages to enhance osteogenic potential via IL-6/GP130/STAT3 signaling. Ann Transl Med. 2023;11(2):90. [24] MOREAUX J, HOSE D, KASSAMBARA A, et al. Osteoclast-gene expression profiling reveals osteoclast-derived CCR2 chemokines promoting myeloma cell migration. Blood. 2011;117(4):1280-1290. [25] SCHWARTZ NB, DOMOWICZ MS. Roles of Chondroitin Sulfate Proteoglycans as Regulators of Skeletal Development. Front Cell Dev Biol. 2022;10:745372. [26] INDIRAN V, JAGANNATHAN D. Osgood-Schlatter Disease. N Engl J Med. 2018;378(11):e15. [27] GUAN P, LIU C, XIE D, et al. Exosome-loaded extracellular matrix-mimic hydrogel with anti-inflammatory property Facilitates/promotes growth plate injury repair. Bioact Mater. 2022;10:145-158. [28] ZHOU FH, FOSTER BK, ZHOU XF, et al. TNF‐α mediates p38 MAP kinase activation and negatively regulates bone formation at the injured growth plate in rats. J Bone Miner Res. 2006;21(7):1075-1088. [29] ARASAPAM G, SCHERER M, COOL JC, et al. Roles of COX‐2 and iNOS in the bony repair of the injured growth plate cartilage. J Cell Biochem. 2006;99(2):450-461. [30] CHUNG R, COOL JC, SCHERER MA, et al. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J Leukoc Biol. 2006;80(6):1272-1280. [31] LEE MA, NISSEN TP, OTSUKA NY. Utilization of a murine model to investigate the molecular process of transphyseal bone formation. J Pediatr Orthop. 2000;20(6):802-806. [32] LIU ES, RAIMANN A, CHAE BT, et al. c-Raf promotes angiogenesis during normal growth plate maturation. Development. 2016;143(2): 348-355. [33] RIBATTI D, D’AMATI A. Bone angiocrine factors. Front Cell Dev Biol. 2023;11:1244372. [34] SU YW, CHUNG R, RUAN CS, et al. Neurotrophin-3 Induces BMP-2 and VEGF Activities and Promotes the Bony Repair of Injured Growth Plate Cartilage and Bone in Rats. J Bone Miner Res. 2016;31(6):1258-1274. [35] PREIN C, BEIER F. ECM signaling in cartilage development and endochondral ossification. Curr Top Dev Biol. 2019;133:25-47. [36] SALHOTRA A, SHAH HN, LEVI B, et al. Mechanisms of bone development and repair. Nat Rev Mol Cell Biol. 2020;21(11):696-711. [37] TUCKERMANN J, ADAMS RH. The endothelium-bone axis in development, homeostasis and bone and joint disease. Nat Rev Rheumatol. 2021;17(10):608-620. [38] KUSUMBE AP, RAMASAMY SK, ADAMS RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014; 507(7492):323-328. [39] ZHANG M, AHN W, KIM S, et al. Endothelial precursor cells stimulate pericyte-like coverage of bone marrow-derived mesenchymal stem cells through platelet-derived growth factor-BB induction, which is enhanced by substance P. Microcirculation. 2017;24(8):e12394. [40] FANG J, HUANG X, HAN X, et al. Endothelial progenitor cells promote viability and nerve regenerative ability of mesenchymal stem cells through PDGF-BB/PDGFR-β signaling. Aging (Albany NY). 2020;12(1):106-121. [41] GUZMAN RA, MARUYAMA M, MOEINZADEH S, et al. The effect of genetically modified platelet-derived growth factor-BB over-expressing mesenchymal stromal cells during core decompression for steroid-associated osteonecrosis of the femoral head in rabbits. Stem Cell Res Ther. 2021;12(1):503. [42] WALIA B, LINGENHELD E, DUONG L, et al. A novel role for cathepsin K in periosteal osteoclast precursors during fracture repair. Ann N Y Acad Sci. 2018;1415(1):57-68. [43] CUI Z, WU H, XIAO Y, et al. Endothelial PDGF-BB/PDGFR-β signaling promotes osteoarthritis by enhancing angiogenesis-dependent abnormal subchondral bone formation. Bone Res. 2022;10(1):58. [44] SHEN Z, DONG W, CHEN Z, et al. Total flavonoids of Rhizoma Drynariae enhances CD31hiEmcnhi vessel formation and subsequent bone regeneration in rat models of distraction osteogenesis by activating PDGFBB/VEGF/RUNX2/OSX signaling axis. Int J Mol Med. 2022;50(3):112. [45] ZHEN G, DAN Y, WANG R, et al. An antibody against Siglec-15 promotes bone formation and fracture healing by increasing TRAP+ mononuclear cells and PDGF-BB secretion. Bone Res. 2021;9(1):47. [46] GAO BO, DENG R, CHAI YU, et al. Macrophage-lineage TRAP+ cells recruit periosteum-derived cells for periosteal osteogenesis and regeneration. J Clin Investig. 2019;129(6):2578-2594. [47] SUN FF, HU PF, XIONG Y, et al. Tricetin protects rat chondrocytes against IL-1β-induced inflammation and apoptosis. Oxid Med Cell Longev. 2019;2019:4695381 [48] SHAW N, ERICKSON C, BRYANT SJ, et al. Regenerative medicine approaches for the treatment of pediatric physeal injuries. Tissue Eng Part B Rev. 2018;24(2):85-97. [49] AZARPIRA MR, SHAHCHERAGHI GH, AYATOLLAHI M, et al.Tissue engineering strategy using mesenchymal stem cell-based chitosan scafolds in growth plate surgery: A preliminary study in rabbits. Orthop Traumatol Surg Res. 2015;101(5):601. [50] CUNNIFFE GM, DÍAZ-PAYNO PJ, RAMEY JS, et al. Growth plate extracellular matrix-derived scaffolds for large bone defect healing. Eur Cell Mater. 2017:33:130-142. [51] FAN M, QIANG L, WANG Y, et al. 3D bioprinted hydrogel/polymer scaffold with factor delivery and mechanical support for growth plate injury repair. Front Bioeng Biotechnol. 2023;11:1210786. [52] QIANG L, FAN M, WANG Y, et al. Injectable hydrogel loaded with bilayer microspheres to inhibit angiogenesis and promote cartilage regeneration for repairing growth plate injury. Front Bioeng Biotechnol. 2023;11:1181580. [53] LI W, XU R, HUANG J, et al. Treatment of rabbit growth plate injuries with oriented ECM scaffold and autologous BMSCs. Sci Rep. 2017;7: 44140. [54] 王香港,万谦,刘贺,等.组织工程软骨在生长板损伤修复治疗中的作用及特点[J].中国组织工程研究,2021,25(28):4539-4545. [55] LI L, HUI JHP, GOH JCH, et al. Chitin as a scaffold for mesenchymal stem cells transfers in the treatment of partial growth arrest. J Pediatr Orthop. 2004;24(2):205-210. [56] MCCARTY RC, XIAN CJ, GRONTHOS S, et al. Application of autologous bone marrow derived mesenchymal stem cells to an ovine model of growth plate cartilage injury. Open Orthop J. 2010;4:204. [57] SCHMITT JF, HUA SK, ZHENG Y, et al. Sequential differentiation of mesenchymal stem cells in an agarose scaffold promotes a physis‐like zonal alignment of chondrocytes. J Orthop Res. 2012;30(11):1753-1759. [58] CLARK A, HILT JZ, MILBRANDT TA, et al. Treating proximal tibial growth plate injuries using poly (lactic-co-glycolic acid) scaffolds. BioResearch Open Access. 2015;4(1):65-74. [59] SONG MH, YOO WJ, CHO TJ, et al. In vivo response of growth plate to biodegradable Mg-Ca-Zn alloys depending on the surface modification. Int J Mol Sci. 2019;20(15):3761. [60] GUAN P, JI Y, KANG X, et al. Biodegradable Dual-Cross-Linked Hydrogels with Stem Cell Differentiation Regulatory Properties Promote Growth Plate Injury Repair via Controllable Three-Dimensional Mechanics and a Cartilage-like Extracellular Matrix. ACS Appl Mater Interfaces. 2023;15(7):8986-8998. [61] VAN DONKELAAR CC, HUISKES R. The PTHrP-Ihh feedback loop in the embryonic growth plate allows PTHrP to control hypertrophy and Ihh to regulate proliferation. Biomech Model Mechanobiol. 2007;6(1-2): 55-62. [62] WANG W, RIGUEUR D, LYONS KM. TGFβ as a gatekeeper of BMP action in the developing growth plate. Bone. 2020;137:115439. [63] ZHANG X, SICLARI VA, LAN S, et al. The critical role of the epidermal growth factor receptor in endochondral ossification. J Bone Miner Res. 2011;26(11):2622-2633. [64] WEI Y, LUO L, GUI T, et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci Transl Med. 2021;13(576):eabb3946. [65] 姚裕斌,魏刚,丁婕,等.可注射水凝胶微球治疗骨关节炎的实验研究[J].中国修复重建外科杂志,2023,37(8):918-928. [66] XIONG J, ONAL M, JILKA RL, et al. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235-1241. |
| [1] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [2] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [3] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [4] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [5] | Liu Haoyang, Xie Qiang, Shen Mengran, Ren Yansong, Ma Jinhui, Wang Bailiang, Yue Debo, Wang Weiguo . Application, research hotspots, and shortcomings of degradable zinc-based alloys in bone defect repair and reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 839-845. |
| [6] | Guo Zhao, Zhuang Haoyan, Shi Xuewen. Role of exosomes derived from mesenchymal stem cells in treatment of colorectal cancer [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7872-7879. |
| [7] | Huang Haina, Yu Yanrong, Bi Jian, Huang Miao, Peng Weijie. Epigenetic characteristics of hepatogenic differentiation of mesenchymal stem cells in three-dimensional culture [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7848-7855. |
| [8] | Liu Lu, Zhong Chang, Yu Xin, Ren Chenyuan, Gong Yangyang, Zhou Ping, Wang Yingbin. Academic progress and clinical application of in vitro synthetic microenvironment to promote maturation of human pluripotent stem cell-derived cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7856-7862. |
| [9] | Ji Long, Chen Ziyang, , Jin Pan, Kong Xiangkui, Pu Rui, . Lipophagy, exercise intervention and prevention and treatment of nonalcoholic fatty liver disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7611-7619. |
| [10] | Yan Jing, Qin Qiujun, Li Fen, Zhou Jun, Ding Yuanyuan, Jin Chunlin. A systematic review of osteoporosis-specific quality-of-life scales [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7649-7655. |
| [11] | Su Yongkun, Sun Hong, Liu Miao, Yang Hua, Li Qingsong. Development of novel antioxidants and antioxidant combination carried by nano-hydrogel systems in treatment of intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7376-7384. |
| [12] | Yi Xiaoding, Zhang Di, Guo Hong, Qing Liang, Zhao Tianyu. Decellularized tendon scaffold: a biomedical material for tendon injury repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7385-7392. |
| [13] | Li Zhongzheng, Chen Zhenghao, Tang Ziyou, Lou Kaiyang, Zhang Rui, Liu Qi, Zhao Na, Yang Kun. Effects of scaffold materials combined with biological factors on biological characteristics of dental follicle cell proliferation and osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7405-7414. |
| [14] | Zhu Jiaping, Gao Bo, Lou Chunbiao, Yang Fengyong, Yang Kun. Monomeric traditional Chinese medicine in the treatment of rheumatoid arthritis: regulation of T cell balance [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6955-6962. |
| [15] | Zhang Pulian, Liu Baoru, Yang Min . Mesenchymal stem cells for treatment of aplastic anemia: inhibiting or activating relevant targets in its pathological evolution [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6800-6810. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||