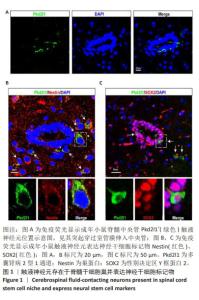
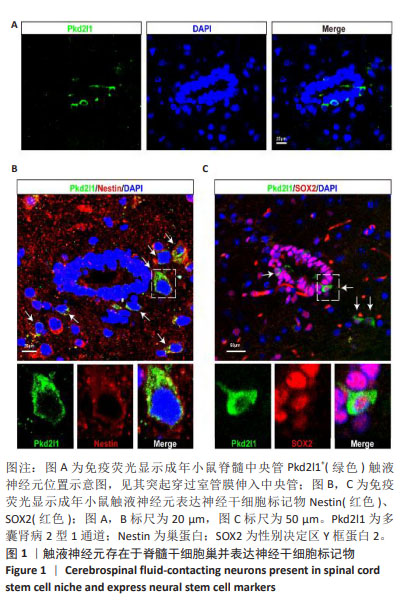
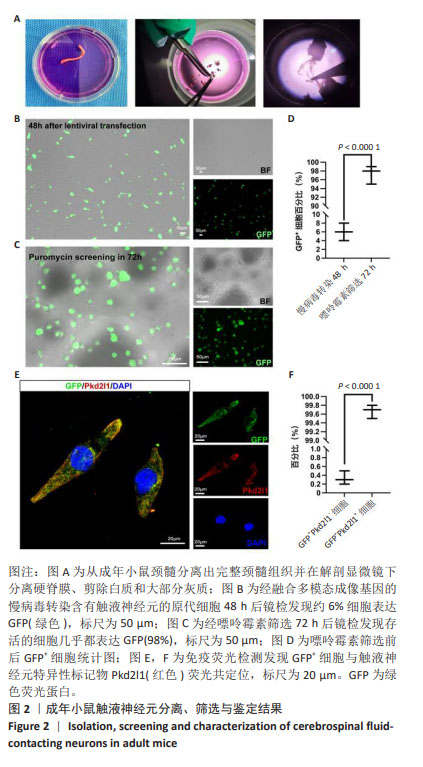
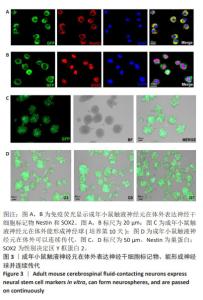
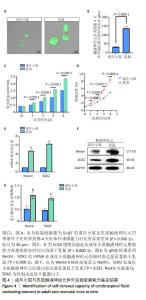
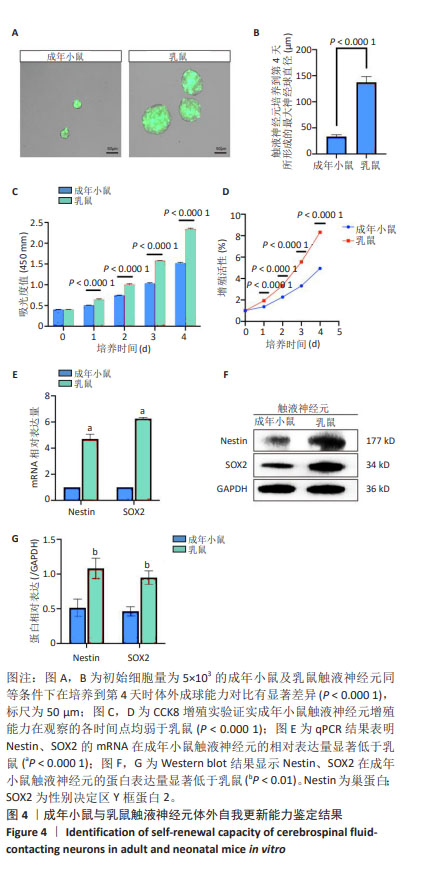
[1] ZIPSER CM, CRAGG JJ, GUEST JD, et al. Cell-based and stem-cell-based treatments for spinal cord injury: evidence from clinical trials. Lancet Neurol. 2022;21(7):659-670.
[2] GILBERT EAB, LAKSHMAN N, LAU KSK, et al. Regulating endogenous neural stem cell activation to promote spinal cord injury repair. Cells. 2022;11(5):846.
[3] CHAKER Z, CODEGA P, DOETSCH F. A mosaic world: puzzles revealed by adult neural stem cell heterogeneity. Wiley Interdiscip Rev Dev Biol. 2016;5(6):640-658.
[4] WYART C, CARBO-TANO M, CANTAUT-BELARIF Y, et al. Cerebrospinal fluid-contacting neurons: multimodal cells with diverse roles in the CNS. Nat Rev Neurosci. 2023;24(9):540-556.
[5] BECKER CG, BECKER T, HUGNOT JP. The spinal ependymal zone as a source of endogenous repair cells across vertebrates. Prog Neurobiol. 2018;170:67-80.
[6] ORTS-DEL’IMMAGINE A, TROUSLARD J, AIRAULT C, et al. Postnatal maturation of mouse medullo-spinal cerebrospinal fluid-contacting neurons. Neuroscience. 2017;343:39-54.
[7] PETRACCA Y L, SARTORETTI M M, DI BELLA D J, et al. The late and dual origin of cerebrospinal fluid-contacting neurons in the mouse spinal cord. Development. 2016;143(5):880-891.
[8] STERNBERG JR, PRENDERGAST AE, BROSSE L, et al. Pkd2l1 is required for mechanoception in cerebrospinal fluid-contacting neurons and maintenance of spine curvature. Nat Commun. 2018;9(1):3804.
[9] GERSTMANN K, JURČIĆ N, BLASCO E, et al. The role of intraspinal sensory neurons in the control of quadrupedal locomotion. Curr Biol. 2022;32(11):2442-2253.e4.
[10] CAO L, HUANG M Z, ZHANG Q, et al. The neural stem cell properties of Pkd2l1(+) cerebrospinal fluid-contacting neurons in vivo. Front Cell Neurosci. 2022;16:992520.
[11] WANG S, HE Y, ZHANG H, et al. The neural stem cell properties of PKD2L1(+) cerebrospinal fluid-contacting neurons in vitro. Front Cell Neurosci. 2021;15:630882.
[12] 罗张荣,曹亮,张毅,等.多模态影像分子体外验证触液神经元的神经干细胞特性[J].中国组织工程研究,2023,27(34):5505-5509.
[13] HE Y Q, SHI XX, CHEN L, et al. Cerebrospinal fluid-contacting neurons affect the expression of endogenous neural progenitor cells and the recovery of neural function after spinal cord injury. Int J Neurosci. 2021;131(6):615-624.
[14] JABBOUR P, FEHLINGS M, VACCARO AR, et al. Traumatic spine injuries in the geriatric population. Neurosurg Focus. 2008;25(5):E16.
[15] ELI I, LERNER DP, GHOGAWALA Z. Acute traumatic spinal cord injury. Neurol Clin. 2021;39(2):471-488.
[16] ADAMS PD, JASPER H, RUDOLPH KL. Aging-induced stem cell mutations as drivers for disease and cancer. Cell Stem Cell. 2015;16(6):601-612.
[17] BAILEY KJ, MASLOV AY, PRUITT SC. Accumulation of mutations and somatic selection in aging neural stem/progenitor cells. Aging Cell. 2004;3(6):391-397.
[18] NICAISE AM, WILLIS CM, CROCKER SJ, et al. Stem cells of the aging brain. Front Aging Neurosci. 2020;12:247.
[19] REYNOLDS BA, WEISS S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707-1710.
[20] JAMES S, THEADOM A, ELLENBOGEN R. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56-87.
[21] MCDONALD JW, SADOWSKY C. Spinal-cord injury. Lancet. 2002; 359(9304):417-425.
[22] FOUAD K, POPOVICH PG, KOPP MA, et al. The neuroanatomical-functional paradox in spinal cord injury. Nat Rev Neurol. 2021;17(1): 53-62.
[23] SOFRONIEW MV. Dissecting spinal cord regeneration. Nature. 2018; 557(7705):343-350.
[24] LIU S, CHEN Z. Employing endogenous NSCs to promote recovery of spinal cord injury. Stem Cells Int. 2019;2019:1958631.
[25] BARNABÉ-HEIDER F, GÖRITZ C, SABELSTRÖM H, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010; 7(4):470-482.
[26] MALIK SC, CHU YH, SCHACHTRUP C. Pointing fingers at blood contact: mechanisms of subventricular zone neural stem cell differentiation. Neural Regen Res. 2023;18(1):137-138.
[27] OBERNIER K, ALVAREZ-BUYLLA A. Neural stem cells:origin, heterogeneity and regulation in the adult mammalian brain. Development. 2019;146(4):dev156059.
[28] JOHANSSON CB, MOMMA S, CLARKE DL, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1):25-34.
[29] MORENO-MANZANO V. Ependymal cells in the spinal cord as neuronal progenitors. Curr Opin Pharmacol. 2020;50:82-87.
[30] STENUDD M, SABELSTRÖM H, LLORENS-BOBADILLA E, et al. Identification of a discrete subpopulation of spinal cord ependymal cells with neural stem cell properties. Cell Rep. 2022;38(9):110440.
[31] SHAH PT, STRATTON JA, STYKEL MG, et al. Single-cell transcriptomics and fate mapping of ependymal cells reveals an absence of neural stem cell function. Cell. 2018;173(4):1045-1057.e9.
[32] BAKER DJ, PETERSEN RC. Cellular senescence in brain aging and neurodegenerative diseases:evidence and perspectives. J Clin Invest. 2018;128(4):1208-1216.
[33] MARIN NAVARRO A, PRONK RJ, VAN DER GEEST AT, et al. p53 controls genomic stability and temporal differentiation of human neural stem cells and affects neural organization in human brain organoids. Cell Death Dis. 2020;11(1):52.
[34] SCAHILL RI, FROST C, JENKINS R, et al. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60(7):989-994.
[35] TERRY DM, DEVINE SE. Aberrantly high levels of somatic LINE-1 expression and retrotransposition in human neurological disorders. Front Genet. 2019;10:1244.
[36] CARRASCO-GARCIA E, MORENO-CUGNON L, GARCIA I, et al. SOX2 expression diminishes with ageing in several tissues in mice and humans. Mech Ageing Dev. 2019;177:30-36.
[37] MUOTRI AR, CHU VT, MARCHETTO MC, et al. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature. 2005;435(7044):903-910.
[38] CODEGA P, SILVA-VARGAS V, PAUL A, et al. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82(3):545-559. |