Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (11): 2285-2293.doi: 10.12307/2025.366
Previous Articles Next Articles
Liuwei Dihuang Wan inhibits oxidative stress in premature ovarian failure mice by regulating intestinal microbiota #br#
#br#
Zhong Jiawen1, 2, 3, Jiang Bo1, 2, 3, Zhang Wenyan1, 2, 3, Li Xiaorong3, 4, Qin Ling1, 2, 3, Gao Ting1, 2, 3
- 1School of Traditional Chinese Medicine, 2Fertility Maintenance Key Laboratory, 3Key Laboratory of Ethnic Medicine Modernization of Ministry of Education, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; 4Ningxia Medical University General Hospital, Yinchuan 750000, Ningxia Hui Autonomous Region, China
-
Received:2024-04-07Accepted:2024-05-10Online:2025-04-18Published:2024-08-10 -
Contact:Li Xiaorong, MD, Associate chief physician, Key Laboratory of Ethnic Medicine Modernization, Ministry of Education, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; Ningxia Medical University General Hospital, Yinchuan 750000, Ningxia Hui Autonomous Region, China -
About author:Zhong Jiawen, Master candidate, School of Traditional Chinese Medicine, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; Fertility Maintenance Key Laboratory of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; Key Laboratory of Ethnic Medicine Modernization, Ministry of Education, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 82260947 (to LXR); the Natural Science Foundation of Ningxia, No. 2023AAC05059 (to LXR)
CLC Number:
Cite this article
Zhong Jiawen, Jiang Bo, Zhang Wenyan, Li Xiaorong, Qin Ling, Gao Ting . Liuwei Dihuang Wan inhibits oxidative stress in premature ovarian failure mice by regulating intestinal microbiota #br#
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
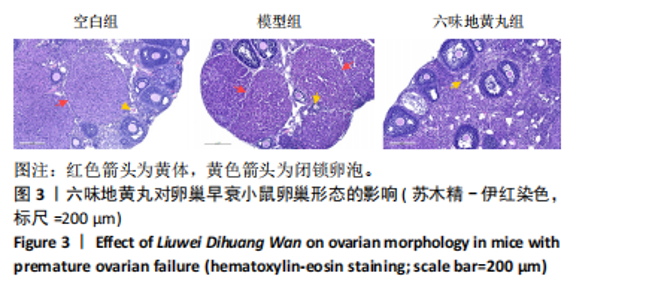
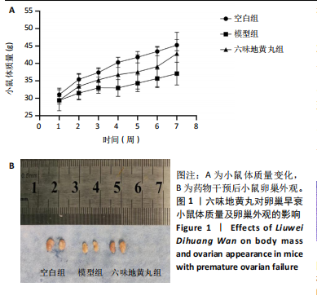
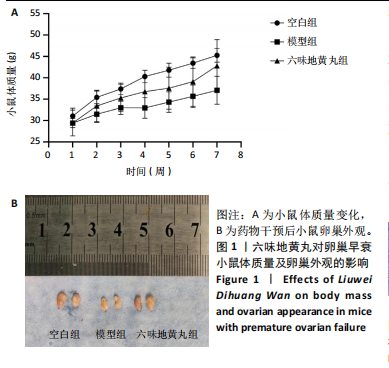
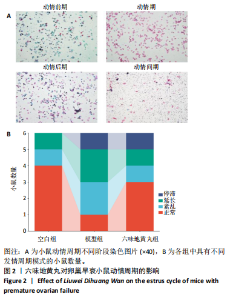
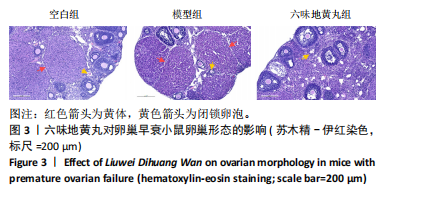
环磷酰胺腹腔注射后连续10 d观察小鼠动情周期,发现小鼠动情周期紊乱,说明环磷酰胺诱导的卵巢早衰小鼠模型建立成功。用六味地黄丸治疗4周后,小鼠的动情周期逐渐恢复。空白组小鼠在整个实验过程中表现出正常的动情周期。正常的ICR雌性小鼠的发情周期为五六天,动情周期各阶段的形态学特征如下[8]:动情前期(P):主要是大量有核上皮细胞;动情期(E):主要是大的、扁平的、无核的角质化鳞状上皮细胞,边缘不规则,无白细胞;动情后期(M):角质化的上皮细胞,具有有核上皮细胞和白细胞;动情间期(D):以大量白细胞为主。 2.4 六味地黄丸对小鼠卵巢形态的影响 苏木精-伊红染色结果显示空白组小鼠卵巢中各种卵泡的形态和数量是正常的,颗粒细胞排列整齐;模型组小鼠卵巢萎缩并出现不规则的卵泡形态,闭锁卵泡增加,黄体数量减少,卵巢颗粒细胞排列松散;在六味地黄丸治疗后出现更多完整的卵泡,闭锁卵泡减少,见图3。"
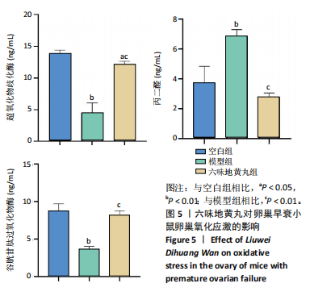
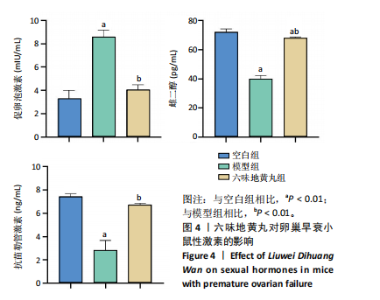
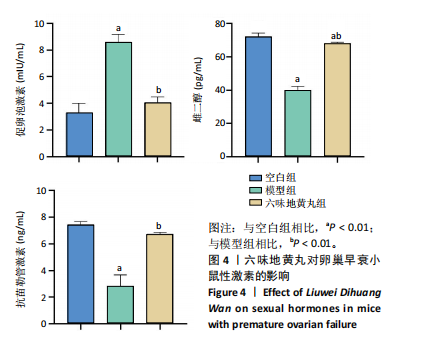
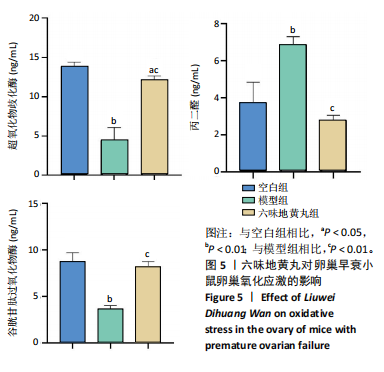
2.6 六味地黄丸抑制卵巢早衰小鼠卵巢氧化应激 丙二醛是脂质过氧化的终产物之一,其含量与机体的氧化应激程度呈正相关[9]。超氧化物歧化酶是一种重要的抗氧化酶,能够清除体内的活性氧自由基,保护细胞免受氧化损伤[10]。谷胱甘肽过氧化物酶作为一种重要的生物标志物,对于评估生物体的氧化应激状态和抗氧化能力具有重要意义。通过ELISA检测这些氧化相关指标,发现与空白组相比,模型组小鼠血清中超氧化物歧化酶和谷胱甘肽过氧化物酶活性显著降低(P < 0.01),丙二醛水平显著上调(P < 0.01),六味地黄丸治疗后显著升高了超氧化物歧化酶和谷胱甘肽过氧化物酶水平(P < 0.01),并降低丙二醛水平(P < 0.01),见图5。"
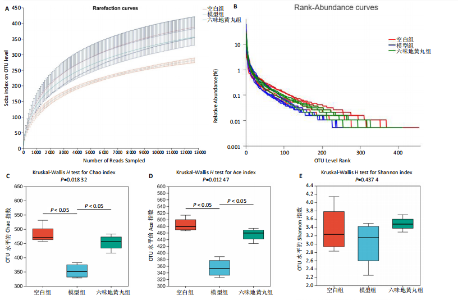
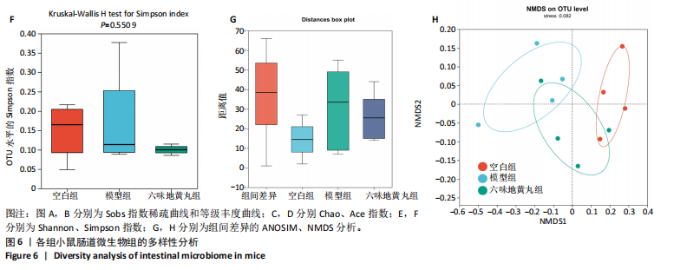
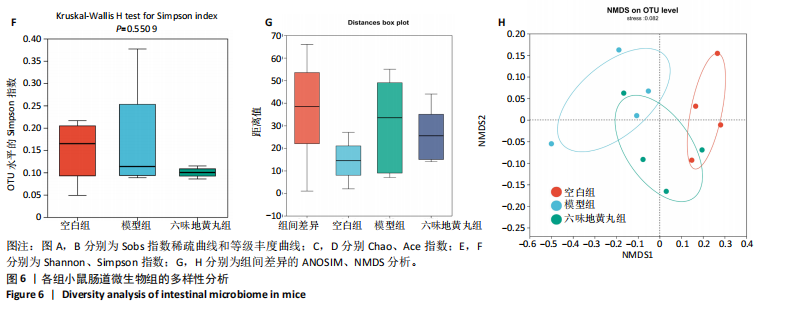
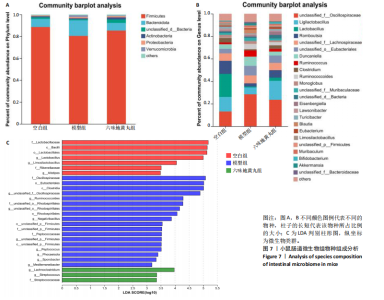
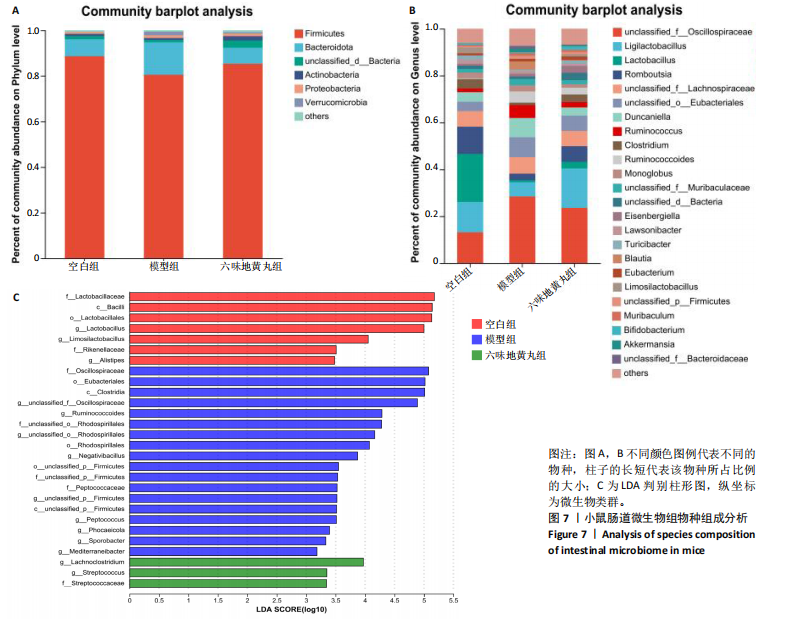
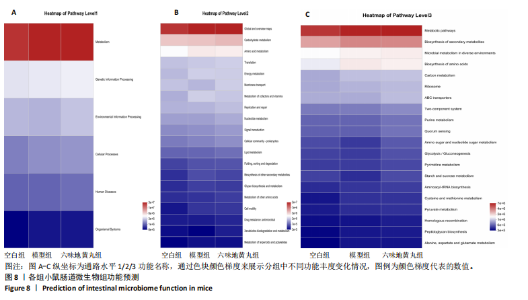
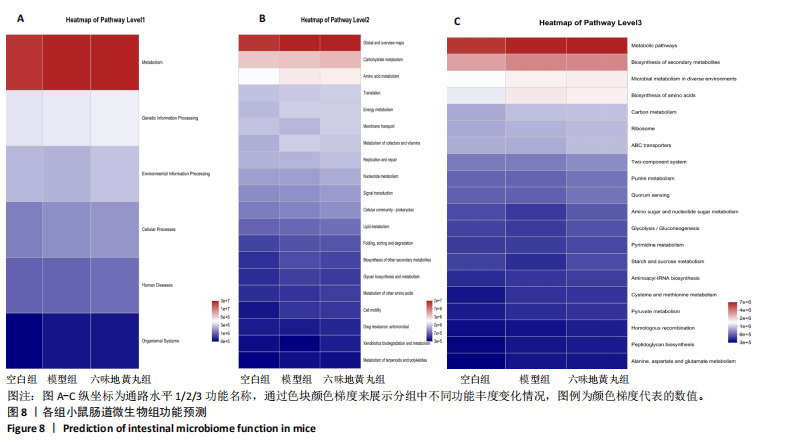
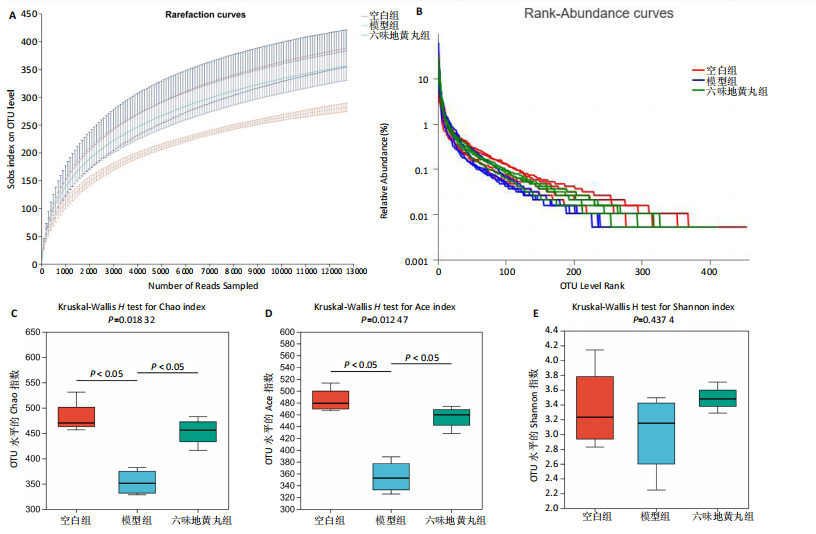
2.7 六味地黄丸减轻卵巢早衰小鼠肠道微生物组失调 2.7.1 肠道微生物组的多样性 六味地黄丸给药治疗4周结束后,收集各组小鼠的粪便样本进行 16S rRNA 测序分析。通过MiSeq测序平台获得双端序列数据。根据不同测序深度下各样品的Sobs指数构建稀疏曲线(图6A)和等级丰度曲线 (图6B)。随着测序深度的增加,稀释曲线和等级丰度曲线的生长方式相似,说明测序数据量和物种丰富度符合进一步分析的条件。α多样性指标Chao和Ace(图6C,D)显示模型组的α多样性显著降低(P < 0.05),六味地黄丸治疗后Chao和Ace指数又显著上升(P < 0.05);根据Shannon和Simpson指数(图6E,F)评估显示3组的物种多样性无差异,说明卵巢早衰显著降低了肠道微生物组的丰富度,而六味地黄丸治疗后又使卵巢早衰小鼠肠道微生物组的丰度显著提升,但3组肠道微生物组的多样性无显著差异。根据组间差异的ANOSIM和NMDS分析(图6G,H)测定了3组肠道微生物组的β多样性,结果表明3组之间存在显著差异。 2.7.2 肠道微生物组的物种变化 为了分析各组肠道微生物的差异并研究六味地黄丸对肠道微生物组的影响,分析了不同水平的肠道微生物组组成。门水平分类分析表明,各类群的粪便菌群主要由厚壁菌门、拟杆菌门组成,约占比80%(图7A);在属水平上,粪便菌群包括颤螺旋菌属、唾液乳杆菌属、乳酸杆菌、罗氏菌属等,这些属的相对丰度在各类群之间各不相同(图7B)。为了进一步确定以分类群形式呈现的生物标志物,使用LEfSe方法对每组进行统计分析,采用LDA分析法估计物种丰富度对差异效应。LDA的临界阈值设定为2.0。结果发现,空白组小鼠中富集了乳酸杆菌科、芽孢杆菌、乳酸杆菌目,卵巢早衰小鼠中富集了颤螺菌科、真细菌目、梭状芽胞杆菌,六味地黄丸组小鼠中也富集了Lachnoclostridium、链球菌、链球菌族(图7C),这一观察结果表明,卵巢早衰小鼠肠道微生物组经六味地黄丸干预后发生了巨大变化。厚壁菌门/拟杆菌门(F/B)比值被认为是肠道微生物组稳态的重要指标[11]。空白组厚壁菌门/拟杆菌门值为15.02,模型组值为5.96,六味地黄丸组值为15.60,模型组相比于空白组厚壁菌门/拟杆菌门值显著下降,六味地黄丸干预后又有所上升,说明六味地黄丸有效地调节了卵巢早衰小鼠肠道微生物组的失调。 2.7.3 肠道微生物组的功能预测 通过PICRUSt2功能预测分析3组小鼠肠道微生物组的基因功能和组成的潜在差异。首先从通路一级分类确定了主要功能,包括新陈代谢、遗传信息处理、环境信息处理、细胞过程、人类疾病、生物体系统(图8A)。KEGG的进一步功能分析涉及全局和概述图谱、碳水化合物代谢、氨基酸代谢、翻译、能量代谢、膜运输、辅因子和维生素代谢等,功能丰度前20见图8B。更详细的功能体现在代谢途径、次生代谢产物的生物合成、不同环境中的微生物代谢、氨基酸的生物合成、碳代谢、核糖体、ABC转运蛋白、双组分调节系统中(图8C)。这些通路的变化与卵巢早衰的发病机制密切相关。"
| [1] 苏比努尔·买买提,尼比热·阿布都瓦依提,易金玲.自身免疫性卵巢早衰发病机制的研究进展[J].妇儿健康导刊,2023,2(9):18-20+37. [2] 柳晓亮,刘一鸣,苏原,等.卵巢早衰中西医病因病机及治疗研究进展[J].长春中医药大学学报,2023,39(7):811-816. [3] 杨婷,王晓红,李怡.褪黑素对早发性卵巢功能不全的临床疗效及机制研究[J].实用临床医药杂志,2023,27(16):75-78+83. [4] MIKHAEL S, PUNJALA-PATEL A, GAVRILOVA-JORDAN L.Hypothalamic-pituitary-ovarian axis disorders impacting femalefertility. Biomedicines. 2019;7(1):5. [5] PARIDA S, SHARMA D. The Microbiome-Estrogen Connection and Breast Cancer Risk. Cells. 2019;8(12):1642. [6] 游婷. 粪菌移植对D-半乳糖诱导的衰老大鼠模型抗氧化功能及肠道菌群影响的研究[D].泸州:西南医科大学,2023. [7] 李慧,王双杰,叶雪,等.卵巢早衰动物模型的建立[J].中国医药生物技术,2020,15(6):572-576. [8] 丁玉龙,孙莉,李丽亚.小鼠阴道涂片三种染色方法比较[J].实验动物科学,2010,27(1):67-69. [9] 张万松.骨髓间充质干细胞(BMSCs)来源的外泌体对睾丸缺血再灌注损伤的保护作用[D].广州:南方医科大学,2018. [10] 关博.哈尔滨市鸡饲料中DON和ZEA的调查及其对鸡脾淋巴细胞毒性的影响[D].哈尔滨:东北农业大学,2013. [11] 赵玉兰.急性敌敌畏暴露致肉鸡小脑自噬及盲肠菌群改变的相关性分析[D].南昌:江西农业大学,2021. [12] JANKOWSKA K. Premature ovarian failure. Prz Menopauzalny. 2017;16(2): 51-56. [13] Bai X, Wang S. Signaling pathway intervention in premature ovarian failure. Front Med (Lausanne). 2022;9:999440. [14] Armeni E, Paschou SA, Goulis DG, et al. Hormone therapy regimens for managing the menopause and premature ovarian insufficiency. Best Pract Res Clin Endocrinol Metab. 2021;35(6):101561. [15] 张艳. 归逍方加减联合人工周期治疗肾虚肝郁证型卵巢早衰闭经49例观察[J].安徽医药,2022,26(1):192-196. [16] MOINI JAZANI A, NASIMI DOOST AZGOMI H, NASIMI DOOST AZGOMI A, et al. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS). Daru. 2019;27(2):863-877. [17] 李红波,王小琴,熊飞,等.六味地黄丸对D-半乳糖致衰老大鼠抗氧化功能影响的实验研究[J].中国中医药科技,2019,26(1):31-33. [18] 梁秋峰,曹云桂,陈奇.六味地黄丸治疗卵巢早衰的研究进展[J].医学综述,2019,25(17):3508-3512. [19] MELEKOGLU R, CIFTCI O, ERASLAN S, et al. Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J Ovarian Res. 2018;11(1):33. [20] PARIDA S, SHARMA D. The microbiome-estrogen connection and breast cancer risk. Cells.2019;8(12):1642. [21] MIKHAEL S, PUNJALA-PATEL A, GAVRILOVA-JORDAN L. Hypothalamic-pituitary-ovarian axis disorders impacting female fertility. Biomedicines. 2019;7(1):5. [22] HAMILTON KJ, HEWITT SC, ARAO Y, et al. Estrogen hormone biology. Curr Top Dev Biol. 2017;125:109-146. [23] TILG H, ZMORA N, ADOLPH TE, et al. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020;20(1):40-54. [24] LINDHEIM L, BASHIR M, MÜNZKER J, et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS One. 2017;12(1):e0168390. [25] QI X, YUN C, PANG Y, et al. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes. 2021;13(1):1-21. [26] JIANG L, FEI H, TONG J, et al. Hormone replacement therapy reverses gut microbiome and serum metabolome alterations in premature ovarian insufficiency. Front Endocrinol (Lausanne). 2021;12:794496. [27] WU J, ZHUO Y, LIU Y, et al. Association between premature ovarian insufficiency and gut microbiota. BMC Pregnancy Childbirth. 2021;21(1):418. [28] WEBBER L, DAVIES M, ANDERSON R, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31: 926-937. [29] AGARWAL A, SALEH RA, BEDAIWY MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829-843. [30] XIE T, YE W, LIU J, et al. The emerging key role of Klotho in the hypothalamus-pituitary-ovarian axis. Reprod Sci. 2021;28(2):322-331. [31] 董若曦.淫羊藿苷抑制氧化应激改善大鼠化疗损伤性卵巢早衰的机制研究[D].上海:上海中医药大学,2019. [32] 潘晓. 番茄红素通过肠-肝轴预防环磷酰胺致小鼠肠道损伤的机制研究[D].保定:河北大学,2023. [33] Helsby NA, Yong M, van Kan M, et al. The importance of both CYP2C19 and CYP2B6 germline variations in cyclophosphamide pharmacokinetics and clinical outcomes. Br J Clin Pharmacol. 2019;85:1925-1934. [34] 陈奕颖.植物乳杆菌Lp2改善脂多糖诱导小鼠炎症反应作用机制研究[D].长春:吉林农业大学,2021. [35] Wang J, Xu J, Han Q, et al. Changes in the vaginal microbiota associated with primary ovarian failure. BMC Microbiol. 2020;20(1):230. [36] Ojo BA, O’Hara C, Wu L, et al. Wheat germ supplementation increases lactobacillaceae and promotes an anti-inflammatory gut milieu in C57BL/6 mice fed a high-fat, high-sucrose diet.J Nutr. 2019;149(7):1107-1115. [37] Gopikrishna T, Suresh Kumar HK, Perumal K, et al. Impact of Bacillus in fermented soybean foods on human health. Ann Microbiol. 2021;71(1):30. [38] Wong-Chew RM, de Castro JA, Morelli L, et al. Gut immune homeostasis: the immunomodulatory role of Bacillus clausii, from basic to clinical evidence. Expert Rev Clin Immunol. 2022;18(7):717-729. [39] 贺乐丽,刘楚鑫,肖毅,等.N-羟乙酰神经氨酸通过调节肠道菌群致小鼠的炎症机制[J/OL].食品科学,1-18[2024-05-06].http://kns.cnki.net/kcms/detail/11.2206.TS.20231229.1411.006.html. [40] 卫钰成,杨敏敏,施琳,等.滇黄精水提物联合间歇性禁食通过调节肠道菌群改善高脂饮食诱导的小鼠肥胖及肝损伤[J].食品与发酵工业, 2022,48(13):91-102. [41] 娄毛闪,于海宁,沈生荣.膳食亚油酸与肠道菌群和慢性代谢性疾病的关系[J].现代医药卫生,2023,39(21):3611-3614+3619. [42] 丁庞华,李军祥,毛堂友,等.微生态制剂治疗溃疡性结肠炎研究进展[J].辽宁中医药大学学报,2018,20(9):140-143. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [3] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| [4] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [5] | Wang Peiguang, Zhang Xiaowen, Mai Meisi, Li Luqian, Huang Hao. Generalized equation estimation of the therapeutic effect of floating needle therapy combined with acupoint embedding on different stages of human knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1565-1571. |
| [6] | Cai Yaohao, Lang Lyu, Li Hong. Assessing the bone mass of the residual alveolar ridge in the first molar for implant placement by cone-beam computed tomography [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1572-1577. |
| [7] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [8] | Su Xiaoyang, Chen Wenting, Fu Yidan, Zhao Yan, Lan Danfeng, Yang Qiuping. Correlation between Mer receptor tyrosine kinase and diabetic peripheral neuropathy in Sprague-Dawley rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1593-1599. |
| [9] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [10] | Li Jun, Gong Jingjing, Sun Guobin, Guo Rui, Ding Yang, Qiang Lijuan, Zhang Xiaoli, Fang Zhanhai . miR-27a-3p promotes the proliferation of human hypertrophic scar fibroblasts by regulating mitogen-activated protein kinase signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1609-1617. |
| [11] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [12] | Jing Ruyi, Chen Yingxin, Cao Lei . Prognosis of deep lamellar keratoplasty versus penetrating keratoplasty in the treatment of stromal corneal dystrophy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1626-1633. |
| [13] | Wang Xuanqiang, Zhang Wenyang, Li Yang, Kong Weiqian, Li Wei, Wang Le, Li Zhongshan, Bai Shi. Effects of chronic exposure to low-frequency pulsed magnetic fields on contractility and morphology of the quadriceps muscle in healthy adults [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1634-1642. |
| [14] | Zhang Yuxin, Yu Cong, Zhang Cui, Ding Jianjun, Chen Yan. Differences in postural control ability between older adults with mild cognitive impairment and those with normal cognition under different single-task and dual-task conditions [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1643-1649. |
| [15] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||