Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (10): 1981-1989.doi: 10.12307/2025.405
Effect of surface roughness of polydimethylsiloxane on osteogenic differentiation of bone marrow mesenchymal stem cells under stretching conditions
Hu Zezun, Yang Fanlei, Xu Hao, Luo Zongping
- Institute of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215000, Jiangsu Province, China
-
Received:2024-02-22Accepted:2024-04-13Online:2025-04-08Published:2024-08-20 -
Contact:Corresponding author: Xu Hao, Associate researcher, Institute of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215000, Jiangsu Province, China Co-corresponding author: Luo Zongping, Professor, Institute of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215000, Jiangsu Province, China -
About author:Hu Zezun, Master candidate, Institute of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215000, Jiangsu Province, China Yang Fanlei, Master candidate, Institute of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215000, Jiangsu Province, China Hu Zezun and Yang Fanlei contributed equally to this article. -
Supported by:National Natural Science Foundation of China, No. 32071307 (to LZP); National Natural Science Foundation of China, No. 11802191 (to XH)
CLC Number:
Cite this article
Hu Zezun, Yang Fanlei, Xu Hao, Luo Zongping. Effect of surface roughness of polydimethylsiloxane on osteogenic differentiation of bone marrow mesenchymal stem cells under stretching conditions[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 1981-1989.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
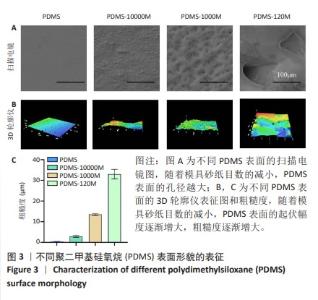
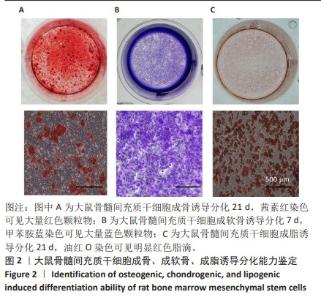
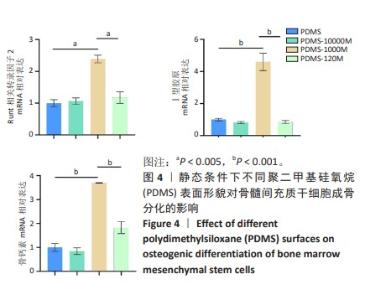
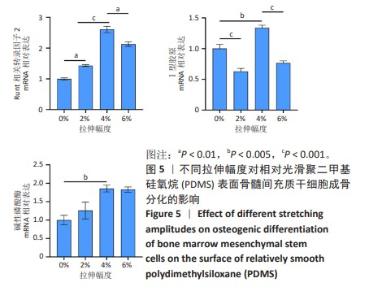
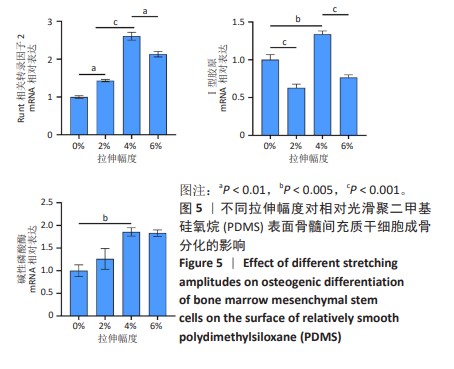
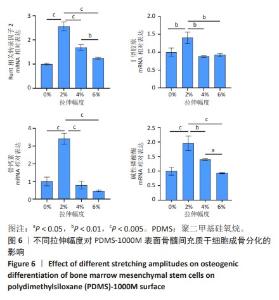
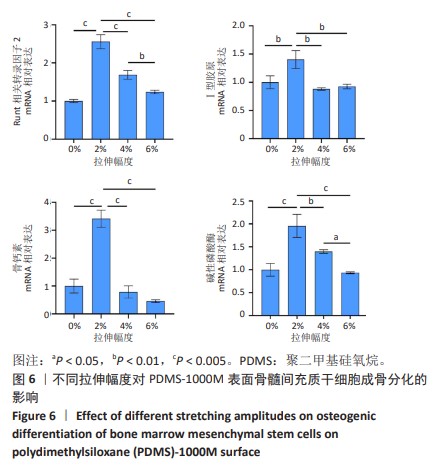
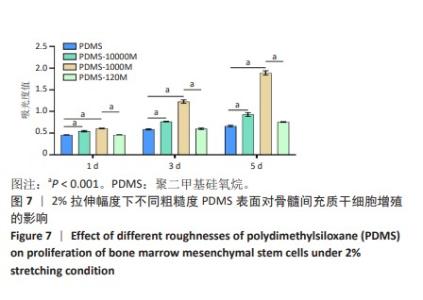
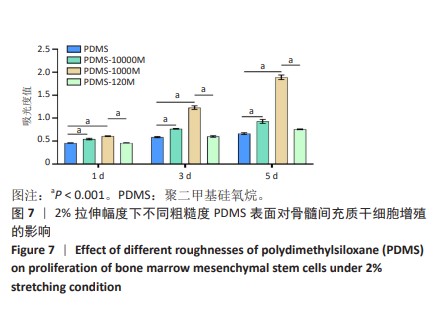
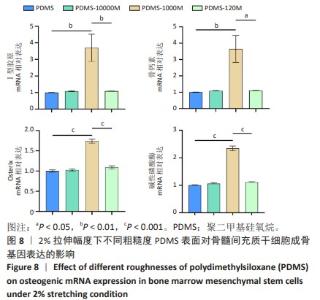
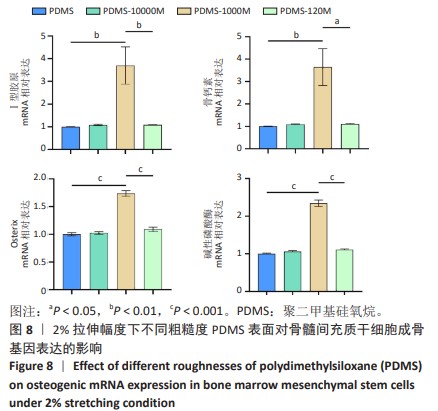
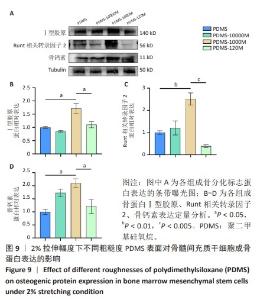
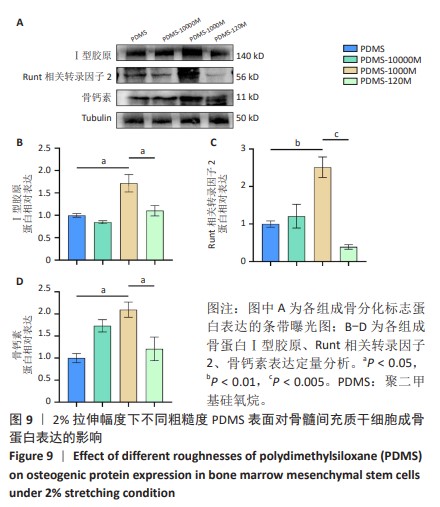
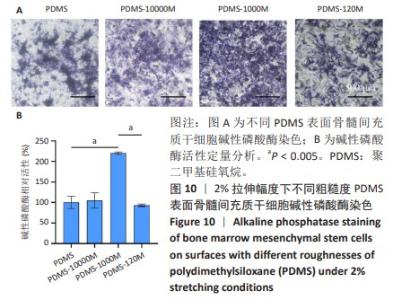
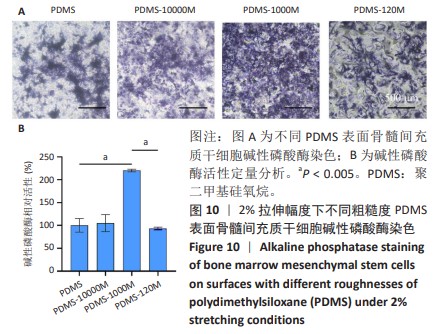
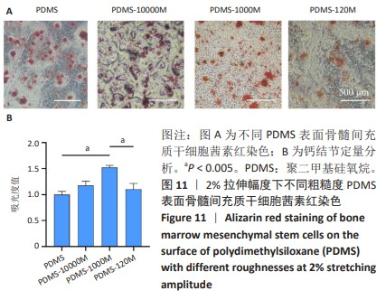
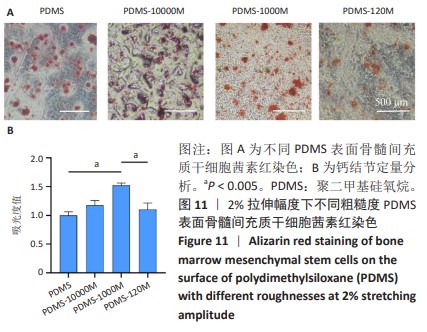
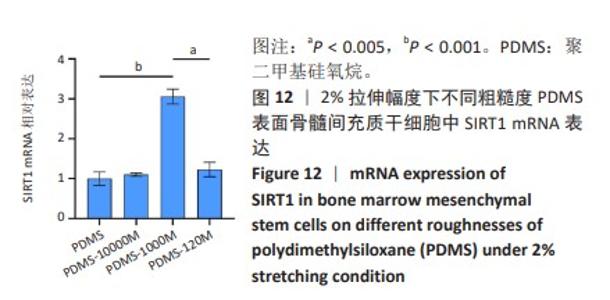
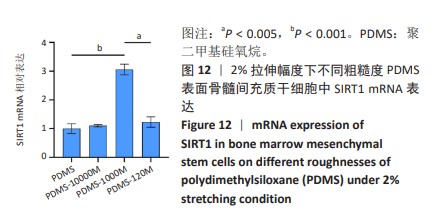
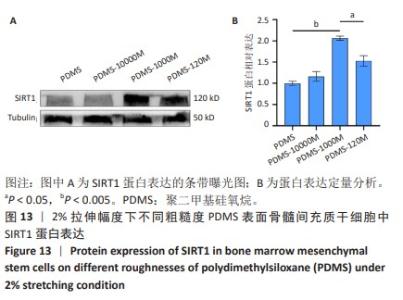
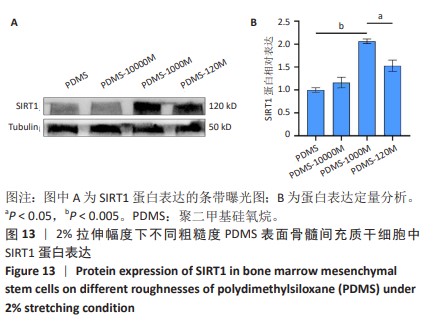
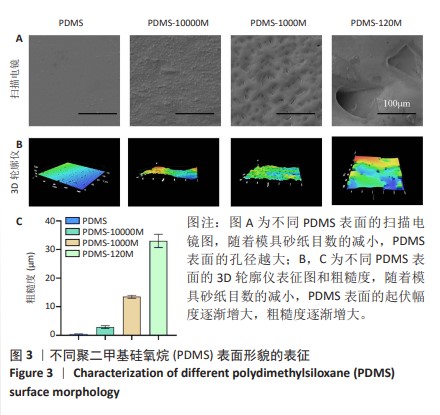
2.2 不同粗糙度的PDMS皿底表征 为了制备具有不同表面形貌的PDMS, 选用120,1 000,10 000目砂纸为模具,标记为PDMS-120M,PDMS-1000M和PDMS-10000M,未与砂纸接触直接与空气接触的PDMS为对照,制作了4种不同表面形貌的PDMS,用于研究不同表面形貌PDMS对骨髓间充质干细胞成骨分化的影响及机制。宏观上看PDMS-120M表面具有明显的起伏,而随着砂纸目数的增大,PDMS表面的起伏越趋近于平坦,未与砂纸接触的PDMS表面较为光滑。通过扫描电镜可以看到PDMS表面随着砂纸目数的减小,孔径越来越大,峰间距逐渐扩大,见图3A。3D轮廓仪可以看到随着砂纸目数的减小,PDMS波峰的平均高度逐渐增大,PDMS、PDMS-10000M、PDMS-1000M和PDMS-120M粗糙度分别为(0.035±0.360),(2.92±1.32),(13.51±2.11),(34.50±6.08) μm, 见图3B,C。"
| [1] CHEN Q, SHOU P, ZHENG C, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7): 1128-1139. [2] CHEN D, LIU S, CHU X, et al. Osteogenic Differentiation Potential of Mesenchymal Stem Cells Using Single Cell Multiomic Analysis. Genes (Basel). 2023;14(10):1871. [3] GAO H, DONG H, ZHENG J, et al. LINC01119 negatively regulates osteogenic differentiation of mesenchymal stem cells via the Wnt pathway by targeting FZD4. Stem Cell Res Ther. 2022;13(1):43. [4] LIU Y, PENG L, LI L, et al. 3D-bioprinted BMSC-laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. Biomaterials. 2021;279:121216. [5] ARTHUR A, GRONTHOS S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int J Mol Sci. 2020;21(24):9759. [6] ERENAY B, SAĞLAM ASY, GARIPCAN B, et al. Bone surface mimicked PDMS membranes stimulate osteoblasts and calcification of bone matrix. Biomater Adv. 2022;142:213170. [7] GUO Q, LIU Y, SUN R, et al. Mechanical stimulation induced osteogenic differentiation of BMSCs through TWIST/E2A/p21 axis. Biosci Rep. 2020;40(5):BSR20193876. [8] ZHANG R, WAN J, WANG H. Mechanical strain triggers differentiation of dental mesenchymal stem cells by activating osteogenesis-specific biomarkers expression. Am J Transl Res. 2019;11(1):233-244. [9] LIN S, LI J, SHAO J, et al. Anisotropic magneto-mechanical stimulation on collagen coatings to accelerate osteogenesis. Colloids Surf B Biointerfaces. 2022;210:112227. [10] SALIFU AA, OBAYEMI JD, UZONWANNE VO, et al. Mechanical stimulation improves osteogenesis and the mechanical properties of osteoblast-laden RGD-functionalized polycaprolactone/hydroxyapatite scaffolds. J Biomed Mater Res A. 2020;108(12):2421-2434. [11] SUMANASINGHE RD, BERNACKI SH, LOBOA EG. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng. 2006;12(12):3459-3465. [12] DONG L, SONG Y, ZHANG Y, et al. Mechanical stretch induces osteogenesis through the alternative activation of macrophages. J Cell Physiol. 2021;236(9):6376-6390. [13] ZHANG D, ZHANG R, SONG X, et al. Uniaxial Cyclic Stretching Promotes Chromatin Accessibility of Gene Loci Associated With Mesenchymal Stem Cells Morphogenesis and Osteogenesis. Front Cell Dev Biol. 2021;9:664545. [14] MAEDA E, ATSUMI Y, ISHIGURO M, et al. Shape-dependent regulation of differentiation lineages of bone marrow-derived cells under cyclic stretch. J Biomech. 2019;96:109371. [15] BAO M, XIE J, HUCK WTS. Recent Advances in Engineering the Stem Cell Microniche in 3D. Adv Sci (Weinh). 2018;5(8):1800448. [16] LIU S, TAO R, WANG M, et al. Regulation of Cell Behavior by Hydrostatic Pressure. Appl Mech Rev. 2019;71(4):0408031-4080313. [17] DE BRUYN H, CHRISTIAENS V, DOORNEWAARD R, et al. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontol 2000. 2017;73(1):218-227. [18] BOYAN BD, BERGER MB, NELSON FR, et al. The Biological Basis for Surface-dependent Regulation of Osteogenesis and Implant Osseointegration. J Am Acad Orthop Surg. 2022;30(13):e894-e898. [19] LU T, SUN Z, JIA C, et al. Roles of irregularity of pore morphology in osteogenesis of Voronoi scaffolds: From the perspectives of MSC adhesion and mechano-regulated osteoblast differentiation. J Biomech. 2023;151:111542. [20] MANIVASAGAM VK, POPAT KC. Hydrothermally treated titanium surfaces for enhanced osteogenic differentiation of adipose derived stem cells. Mater Sci Eng C Mater Biol Appl. 2021;128:112315. [21] LOLLOBRIGIDA M, LAMAZZA L, CAPUANO C, et al. Physical Profile and Impact of a Calcium-Incorporated Implant Surface on Preosteoblastic Cell Morphologic and Differentiation Parameters: A Comparative Analysis. Int J Oral Maxillofac Implants. 2016;31(1):223-231. [22] LI Y, XIAO Y, LIU C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem Rev. 2017;117(5):4376-4421. [23] ZHU GY, LIU YH, LIU W, et al. Surface Epitaxial Nano-Topography Facilitates Biomineralization to Promote Osteogenic Differentiation and Osteogenesis. ACS Omega. 2021;6(33):21792-21800. [24] MONTEIRO NO, CASANOVA MR, FANGUEIRO JF, et al. The biomimetic surface topography ofRubus fruticosusleaves stimulate the induction of osteogenic differentiation of rBMSCs. Biomed Mater. 2023;18(3). doi: 10.1088/1748-605X/acc55f. [25] SARUTA J, SATO N, ISHIJIMA M, et al. Disproportionate Effect of Sub-Micron Topography on Osteoconductive Capability of Titanium. Int J Mol Sci. 2019;20(16):4027. [26] 唐丽灵,潘君,王远亮,等.骨的应力集中效应 [J].医用生物力学, 2002,17(3):189-192. [27] ZHANG L, ZHENG YL, WANG R, et al. Exercise for osteoporosis: A literature review of pathology and mechanism. Front Immunol. 2022; 13:1005665. [28] CHEN X, YAN J, HE F, et al. Mechanical stretch induces antioxidant responses and osteogenic differentiation in human mesenchymal stem cells through activation of the AMPK-SIRT1 signaling pathway. Free Radic Biol Med. 2018;126:187-201. [29] 熊婉琦,李振豪,崔焱,等.生物力学作用对成骨细胞生物特性的影响[J].中国组织工程研究,2024,28(21):3407-3412. [30] 聂文忠,蒋光普,韩笑,等.骨质疏松患者骨水泥强化内固定系统的生物力学特征[J].中国组织工程研究,2023,27(21):3287-3292. [31] SAUVE AA, WOLBERGER C, SCHRAMM VL, et al. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435-465. [32] XU C, WANG L, FOZOUNI P, et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol. 2020;22(10): 1170-1179. [33] HE F, LI Q, SHENG B, et al. SIRT1 Inhibits Apoptosis by Promoting Autophagic Flux in Human Nucleus Pulposus Cells in the Key Stage of Degeneration via ERK Signal Pathway. Biomed Res Int. 2021;2021: 8818713. [34] RADA P, PARDO V, MOBASHER MA, et al. SIRT1 Controls Acetaminophen Hepatotoxicity by Modulating Inflammation and Oxidative Stress. Antioxid Redox Signal. 2018;28(13):1187-1208. [35] PARDO PS, MOHAMED JS, LOPEZ MA, et al. Induction of Sirt1 by mechanical stretch of skeletal muscle through the early response factor EGR1 triggers an antioxidative response. J Biol Chem. 2011; 286(4):2559-2566. [36] YANG L, LI Y, GONG R, et al. The Long Non-coding RNA-ORLNC1 Regulates Bone Mass by Directing Mesenchymal Stem Cell Fate. Mol Ther. 2019;27(2):394-410. [37] LEE SI, PARK KH, KIM SJ, et al. Mechanical stress-activated immune response genes via Sirtuin 1 expression in human periodontal ligament cells. Clin Exp Immunol. 2012;168(1):113-124. [38] LU Y, MA ZX, DENG R, et al. The SIRT1 activator SRT2104 promotes BMP9-induced osteogenic and angiogenic differentiation in mesenchymal stem cells. Mech Ageing Dev. 2022;207:111724. [39] ZHOU L, CHEN X, LIU T, et al. Melatonin reverses H2 O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J Pineal Res. 2015;59(2):190-205. |
| [1] | Sun Xianjuan, Wang Qiuhua, Zhang Jinyi, Yang Yangyang, Wang Wenshuang, Zhang Xiaoqing. Adhesion, proliferation, and vascular smooth muscle differentiation of bone marrow mesenchymal stem cells on different electrospinning membranes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 661-669. |
| [2] | Xu Zhenhua, Li Yanjie, Qin Hewei, Liu Haoyuan, Zhu Bochao, Wang Yupu. Traditional Chinese medicine monomer in treatment of neuroinflammation after spinal cord injury: effects of nuclear transcription factor kappa B signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 590-598. |
| [3] | Tian Jiayu, Li Duohua, Zhang Feng, Feng Hu, Sun Wei. Construction and performance evaluation of polycaprolactone/nanodiamond-phospholipid composite materials [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3380-3387. |
| [4] | Qin Jingjie, Guo Zige, Li Rui, Ma Shiqing, Lu Ruijie, Li Mengjun. Modification with bone forming peptide 1 and polydopamine coating to improve bioactivity of polyetheretherketone surface [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3318-3325. |
| [5] | E Zhengkang, Xin Hongwei, Yu Qingbo, Zhang Yunshuai. miR-192-5p targets CKIP-1 to promote osteogenic differentiation of bone marrow mesenchymal stem cells in osteoporosis patients [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2641-2647. |
| [6] | Huang Liping, Li Hui, Wang Xinge, Wang Rui, Chang Bei, Li Shiting, Lan Xiaorong, Li Guangwen. Effects of microstructured bone implant material surfaces on osteogenic function of MC3T3-E1 osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 1990-1996. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||