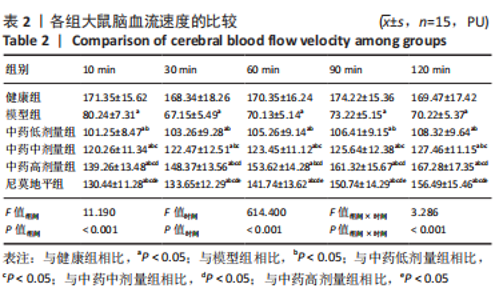
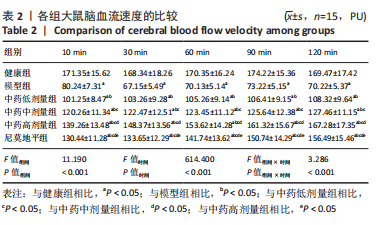
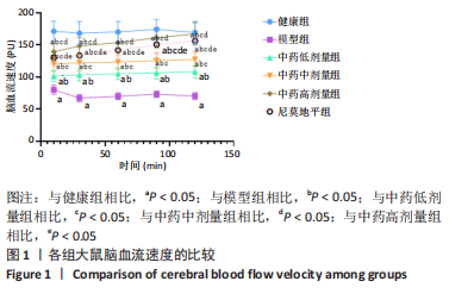
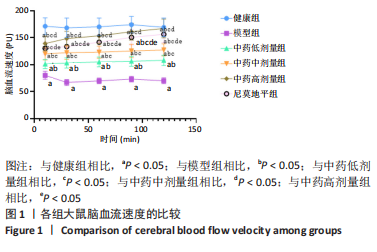
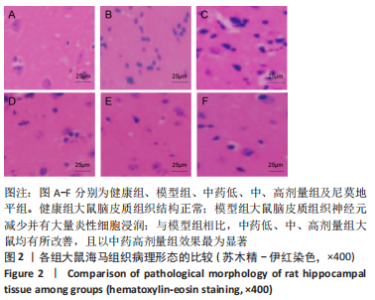
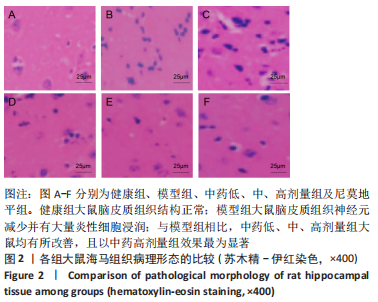
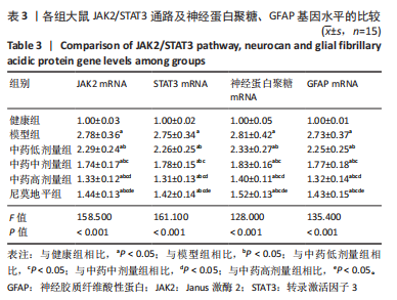
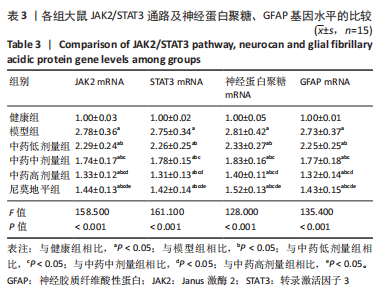
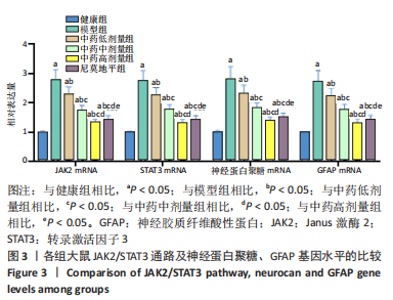
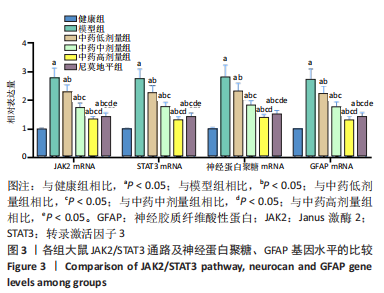
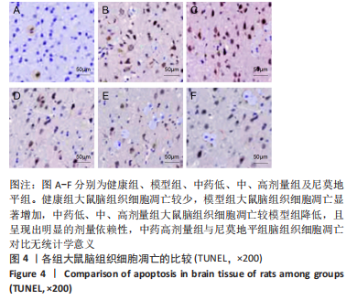
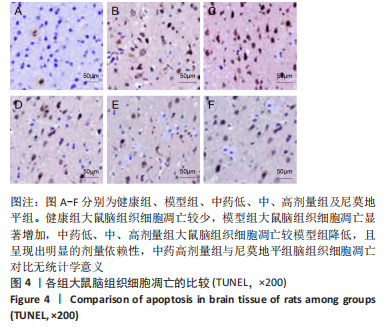
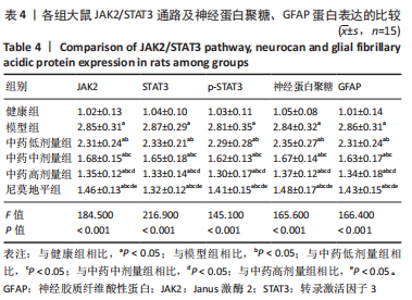
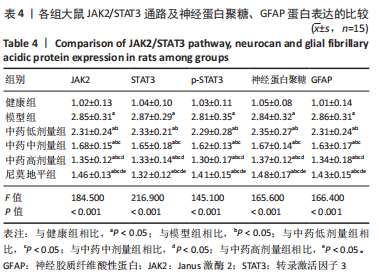
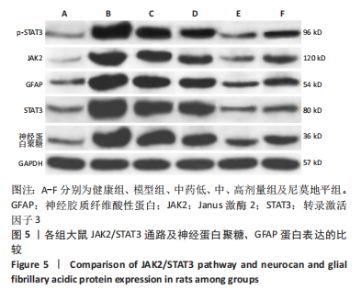
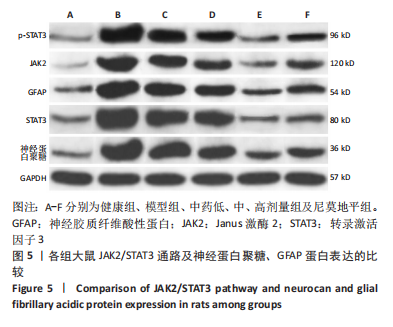
[1] JIN Y, JIBO Z, JINCAO C. The Significance to Determine Factors Related to Postoperative Cerebral Infarction in Patients with Moyamoya Disease. Cerebrovasc Dis. 2020;49(2):233-234.
[2] 叶建宇,孙自玉,胡薇薇.星形胶质细胞在脑梗死中的作用及相关治疗策略[J].浙江大学学报(医学版),2018,47(5):493-498.
[3] 胡洪凭,张洪凯,吴滨.丙泊酚通过PI3K/Akt/mTOR通路对脑外伤大鼠的神经保护作用[J].中国药师,2019,22(7):1215-1219.
[4] 张志鑫,崔应麟,李彦杰,等.14-3-3ε激活NF-κB通路参与氧糖剥夺/再灌注诱导的原代培养大鼠脊髓星形胶质细胞活化[J].神经解剖学杂志,2016, 32(6):698-704.
[5] 刘萍.急性期脑梗死患者血清UCH-L1和GFAP水平及其与病情和神经功能缺损程度的相关性研究[J].实用医院临床杂志,2019,16(2):173-176.
[6] 崔海燕,孙芳云,李捷,等.藏药七十味珍珠丸对帕金森病模型大鼠多巴胺能神经元的保护作用[J].中华中医药杂志,2018,33(6):2616-2619.
[7] 梁源,付珂,王张,等.藏药七十味珍珠丸灌胃给予脑缺血再灌注损伤模型大鼠的量-时-效关系研究[J].中国药房,2019,30(1):94-98.
[8] YI ZA , BO Y, YY A, et al. The bidirectional role of the JAK2/STAT3 signaling pathway and related mechanisms in cerebral ischemia-reperfusion injury. Exp Neurol. 2021;341:113690.
[9] 央金卓玛,吉太加.藏医火灸联合七十味珍珠丸对卒中偏瘫患者肢体功能的影响[J].吉林中医药,2020,40(5):642-646.
[10] 薛岚平,张琴琴. 氧化苦参碱对急性脑梗死大鼠鞘氨醇激酶2及闭合蛋白5的影响[J]. 中华生物医学工程杂志,2019,25(4):422-426.
[11] 梁源,付珂,王张,等.藏药七十味珍珠丸灌胃给予脑缺血再灌注损伤模型大鼠的量-时-效关系研究[J].中国药房,2019,30(1):94-98.
[12] 林珑,陈祥荣,王守森.炎症状态下小胶质细胞引起的脑微循环障碍及其致病作用的研究进展[J].中华神经医学杂志,2022,21(5):511-515.
[13] 郭芳,张拥波.高血压对大脑中动脉闭塞患者软脑膜侧支循环的影响[J].临床和实验医学杂志,2019,18(1):40-43.
[14] 仁欠多杰,宗咏花,次旦多吉.藏药七十味珍珠丸治疗脑梗死的效果及预后探讨[J].中国民族医药杂志,2022,28(5):9-10.
[15] 增太吉.观察藏药七十味珍珠丸治疗脑出血(脑溢血)的临床疗效[J].智慧健康,2019,5(28):179-180.
[16] 高萌.藏药七十味珍珠丸联合银杏叶注射液治疗腔隙性脑梗死的临床疗效[J].中国民族医药杂志,2020,26(11):2-4.
[17] 增太吉.观察藏药七十味珍珠丸治疗脑出血(脑溢血)的临床疗效[J].智慧健康,2019,5(28):179-180.
[18] 梁源. 基于GC-MS和代谢组学的七十味珍珠丸治疗脑缺血BBB损伤的入血成分和作用机制研究[D].成都:成都中医药大学,2019.
[19] 陈红霞,裴晋云,潘锐焕,等.七十味珍珠丸治疗63例脑卒中恢复期患者临床疗效研究[J].时珍国医国药,2018,29(12):2968-2970.
[20] 梁源,孙位军,王张,等.七十味珍珠丸对脑缺血再灌注损伤大鼠血脑屏障的保护作用[J].中成药,2019,41(4):767-773.
[21] 尹小玲,刘勇,张伏军.尼莫地平联合依达拉奉治疗老年急性脑梗死的临床效果[J].中华老年多器官疾病杂志,2019,18(6):430-434.
[22] PANG QM, CHEN SY, XU QJ, et al. Neuroinflammation and Scarring After Spinal Cord Injury: Therapeutic Roles of MSCs on Inflammation and Glial Scar. Front Immunol. 2021;12:751021.
[23] TRAN AP, WARREN PM, SILVER J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 2022;387(3):319-336.
[24] MATTILA OS, ASHTON NJ, KAJ B , et al. Ultra-Early Differential Diagnosis of Acute Cerebral Ischemia and Hemorrhagic Stroke by Measuring the Prehospital Release Rate of GFAP. Clin Chem. 2021;(10):1361-1372.
[25] 张维文,张方圆,方玲,等.早期运动训练对脑梗死后缺血半暗带GAP-43、Nogo-A及GFAP表达的影响[J].解剖学杂志,2020,43(5):407-411+420.
[26] 马冉冉,李健瑞,伏亚红,等.早期强化运动训练与电针治疗对脑缺血再灌注后大鼠神经功能恢复的作用[J].内科急危重症杂志,2019,25(3):227-231.
[27] CHEN B, YANG L, CHEN J, et al. Inhibition of Connexin43 Hemichannels with Gap19 Protect-s Cerebral Ischemia/Reperfusion Injury via the JAK2/STAT3 Pathway in Mice. Brain Res Bull. 2019;146:124-135.
[28] GUO X, SHEN X, YONG Z. MiR-101 Protects Against the Cerebral I/R Injury Through Regulating JAK2/STAT3 Signaling Pathway. Neuropsychiatr Dis Treat. 2021;17:2791-2802.
[29] WANG R, ZHANG S, YANG Z, et al. Mutant erythropoietin enhances white matter repair via the JAK2/STAT3 and C/EBPβ pathway in middle-aged mice following cerebral ischemia and reperfusion. Exp Neurol. 2021;337:113553.
[30] LAN H, SU Y, LIU Y, et al. Melatonin protects circulatory death heart from ischemia/reperfusion injury via the JAK2/STAT3 signalling pathway. Life Sci. 2019; 228:35-46.
[31] 于晓岚,李光楠,赵婷.葛根素对脑梗死大鼠神经功能神经细胞凋亡及Jak2/STAT3信号传送途径的影响[J].山西医药杂志,2020,49(3):243-246.
|