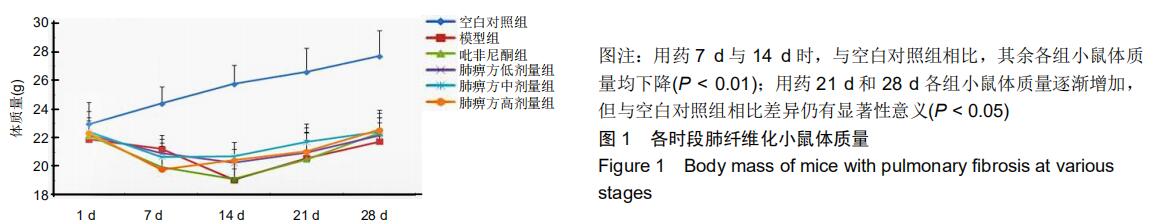
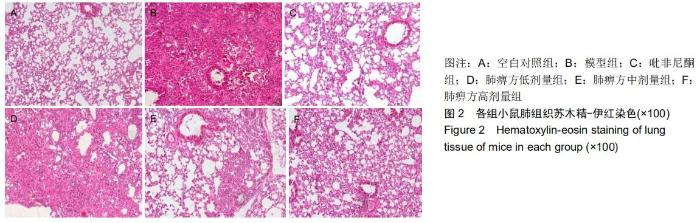
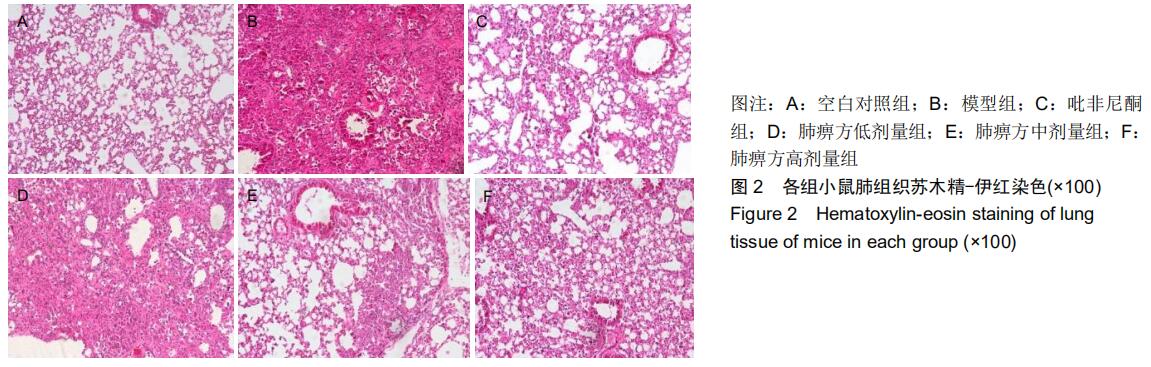
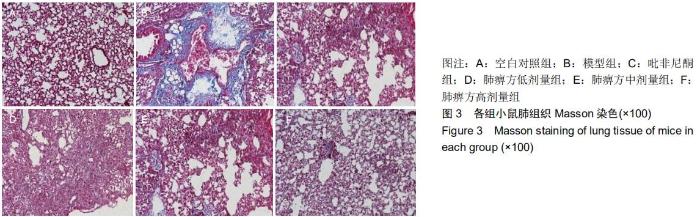
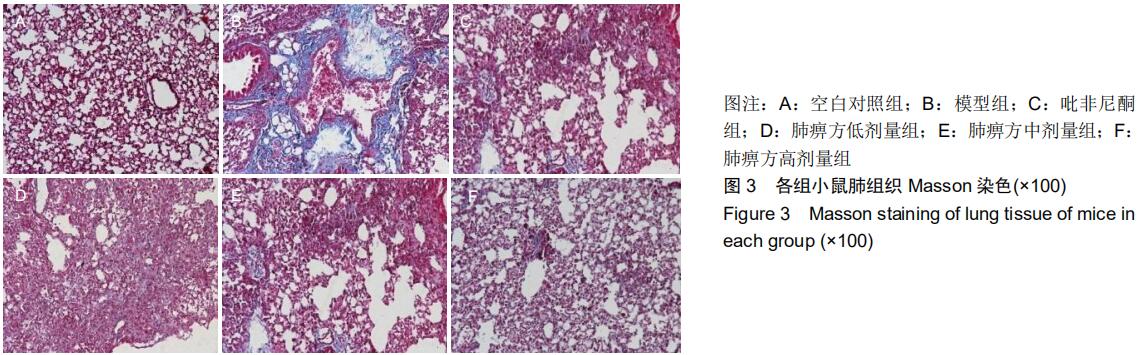
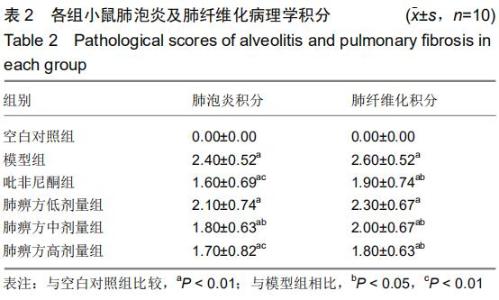
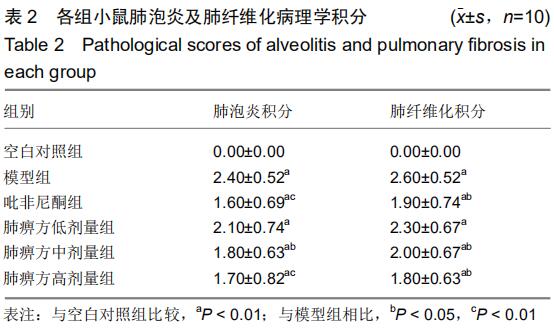
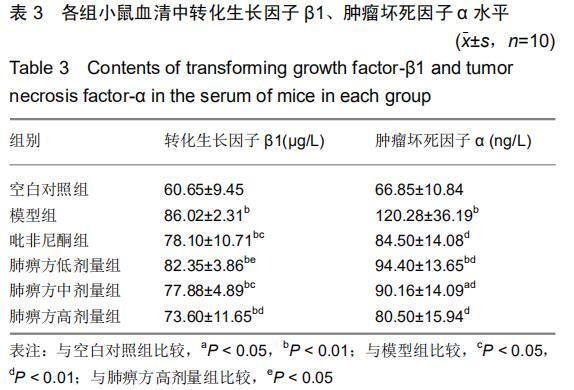
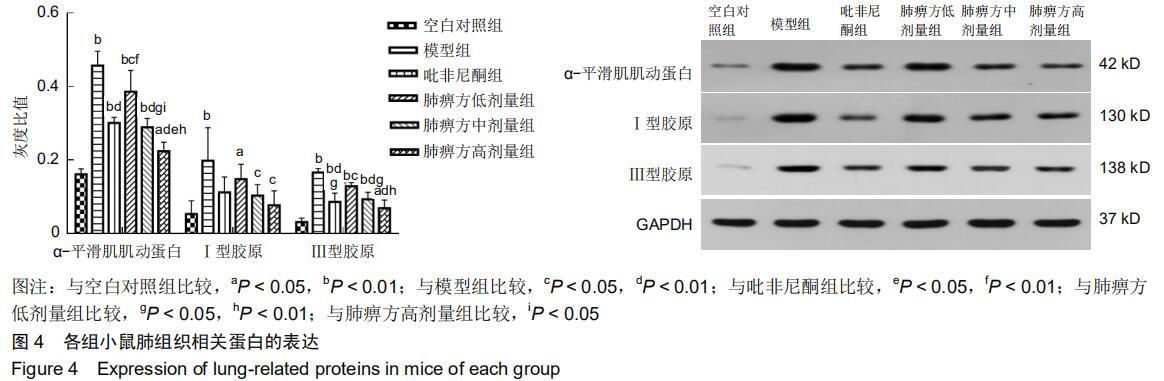
|
[1] GEORGE PM, PATTERSON CM, REED AK, et al. Lung transplantation for idiopathic pulmonary fibrosis.Lancet Respir Med.2019;7(3): 271-282.
[2] MARTINEZ FJ, COLLARD HR, PARDO A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074.
[3] GAO L, JIANG D, GENG J, et al. Hydrogen inhalation attenuated bleomycin-induced pulmonary fibrosis by inhibiting transforming growth factor-β1 and relevant oxidative stress and epithelial-to- mesenchymal transition. Exp Physiol. 2019;104(12):1942-1951.
[4] LI YQ, XIANG MR, RONG R, et al. Inhibitory effect of Glehniae Radix petroleum ether part on TGF-β1-induced epithelial mesenchymal transition in A549 cells. Zhongguo Zhong Yao Za Zhi. 2017;42(9): 1736-1741.
[5] NATHAN SD, BARNETT SD, MORAN B, et al. Interferon gamma-1b as therapy for idiopathic pulmonary fibrosis. An intrapatient analysis. Respiration. 2004;71(1):77-82.
[6] 吴银根,唐斌擎.吴银根肺系疾病中医诊疗思路与经验[M].上海:上海科学技术出版社,2016:198.
[7] 程雪,王丽新,孙鼎,等.基于中医传承辅助系统挖掘吴银根治疗特发性肺纤维化的用药规律[J].中国中医基础医学杂志, 2018,24(11):1562-1564.
[8] 程雪,李少滨,吴银根,等.吴银根治疗间质性肺炎用药规律研究[J].山东中医杂志,2018,37(5):392-394,408.
[9] 程雪,方泓,吴银根.肺痹方对肺纤维化小鼠抗氧化作用的实验研究[J].中国中医急症,2018,27(3):394-397,409.
[10] MAEDA A, HIYAMA K, YAMAKIDO H, et al. Increased expression of platelet-derived growth factor A and insulin-like growth factor-I in BAL cells during the development of bleomycin-induced pulmonary fibrosis in mice. Chest. 1996;109(3):780-786.
[11] 章元沛.药理学实验[M].北京:人民卫生出版社,1996:238.
[12] SZAPIEL SV, ELSON NA, FULMER JD, et al. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis. 1979;120(4):893-899.
[13] CHUA F, GAULDIE J, LAURENT GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 2005;33(1):9-13.
[14] 卢锦辉,张丽,刘子豪,等.博来霉素诱导小鼠肺纤维化模型的建立及评价[J].兰州大学学报(医学版),2019,45(6):37-42.
[15] 李丽娜,王华,周蕾,等.博莱霉素诱导小鼠肺间质纤维化造模方式的选择[J].中国免疫学杂志,2010,26(3):254-257.
[16] 李戎,闫志勇,李文军,等.肺纤维化发生与防治机理的重要因素—细胞因子作用机制研究[J].中国中医药现代远程教育, 2004,2(6):23-26.
[17] SENAVIRATHNA LK, HUANG C, PUSHPARAJ S, et al. Hypoxia and transforming growth factor β1 regulation of long non-coding RNA transcriptomes in human pulmonary fibroblasts. Physiol Rep. 2020; 8(1):e14343.
[18] SUN J, USUNE S, ZHAO Y, et al. Multidrug resistance protein transporter and Ins(1,4,5)P₃-sensitive Ca²+-signaling involved in adenosine triphosphate export via Gq protein-coupled NK₂-receptor stimulation with neurokinin A. J Pharmacol Sci. 2010;114(1):92-98.
[19] KOMMAJOSYULA SP, RANDALL ME, FAINGOLD CL. Inhibition of adenosine metabolism induces changes in post-ictal depression, respiration, and mortality in genetically epilepsy prone rats. Epilepsy Res. 2016;119:13-19.
[20] HIGGINS DF, KIMURA K, BERNHARDT WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117(12):3810-3820.
[21] LIU RM. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal. 2008;10(2):303-319.
[22] GOLAN-GERSTL R, WALLACH-DAYAN SB, AMIR G, et al. Epithelial cell apoptosis by fas ligand-positive myofibroblasts in lung fibrosis. Am J Respir Cell Mol Biol. 2007;36(3):270-275.
[23] LUCATTELLI M, FINESCHI S, SELVI E, et al. Ajulemic acid exerts potent anti-fibrotic effect during the fibrogenic phase of bleomycin lung. Respir Res. 2016;17(1):49.
[24] 张天嵩,吴银根.通补肺络法治疗肺纤维化理论探讨[J].中医杂志, 2002, 43(11):808-810.
[25] 刘晓明,朱雪,张伟.化痰类中药干预肺纤维化模型大鼠肺组织中Nrf2含量的研究[J].时珍国医国药,2017,28(9):2081-2084.
[26] 赵霞,王丽新.特发性肺纤维化治验[J].湖南中医杂志, 2012,28(3):92-93.
[27] 孟丽红,张晓梅,董环,等.养阴益气合剂对肺纤维化大鼠TGF-β/Smad信号通路的影响[J].环球中医药, 2019,12(12):1810-1815.
[28] 牟玮,宋雅琳,张硕,等.冬虫夏草治疗慢性阻塞性肺疾病临床疗效的系统评价[J].中国循证医学杂志,2013,13(11):1373-1381.
[29] 宋建平,刘方州,李伟,等.金匮肾气丸对肺纤维化大鼠组织中肿瘤坏死因子a表达的影响[J].中成药,2006,28(1):78-81.
[30] 薛娟.川芎嗪对人胚肺成纤维细胞系MRC-5细胞增殖及胶原蛋白合成的作用[D].石家庄:河北大学,2014.
|