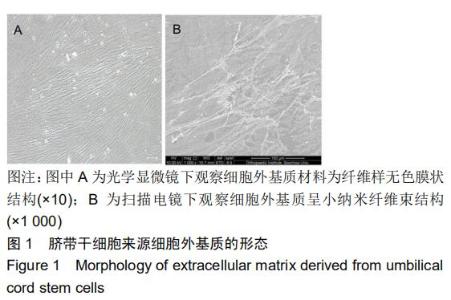
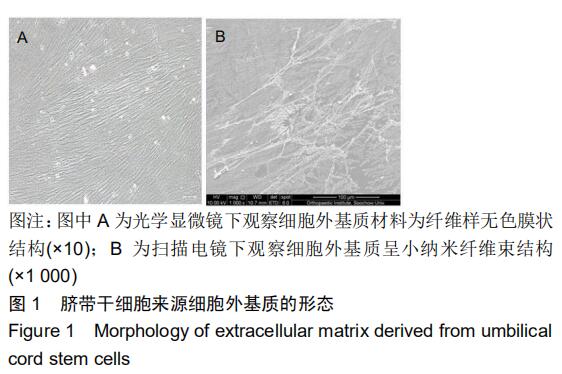
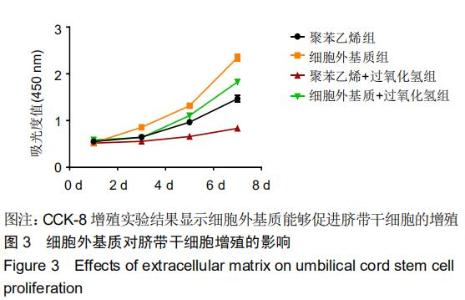
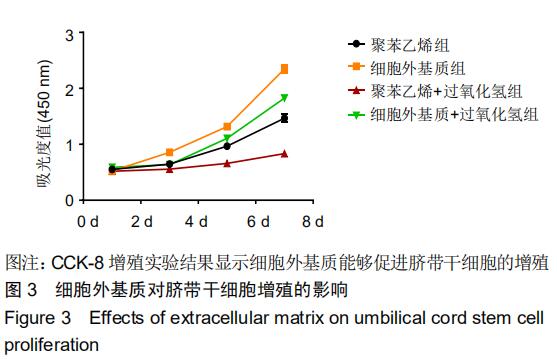
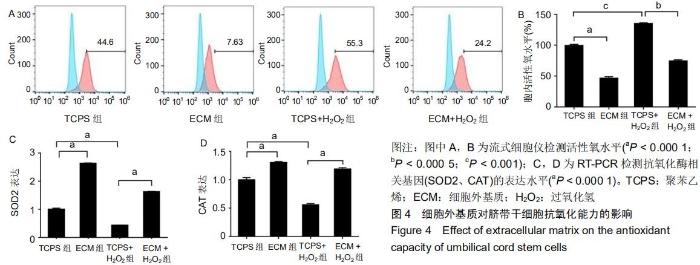
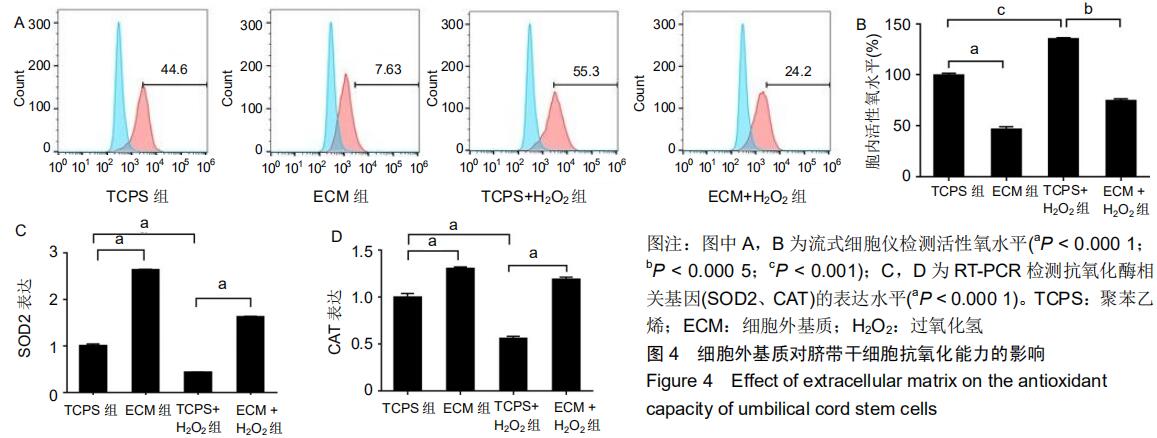
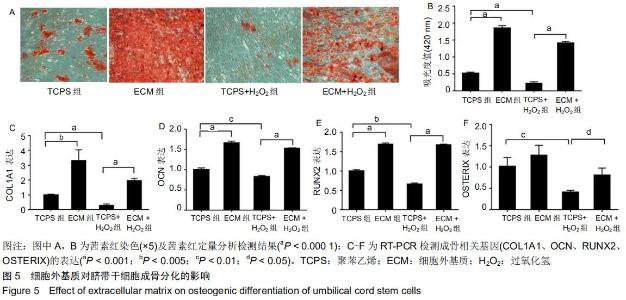
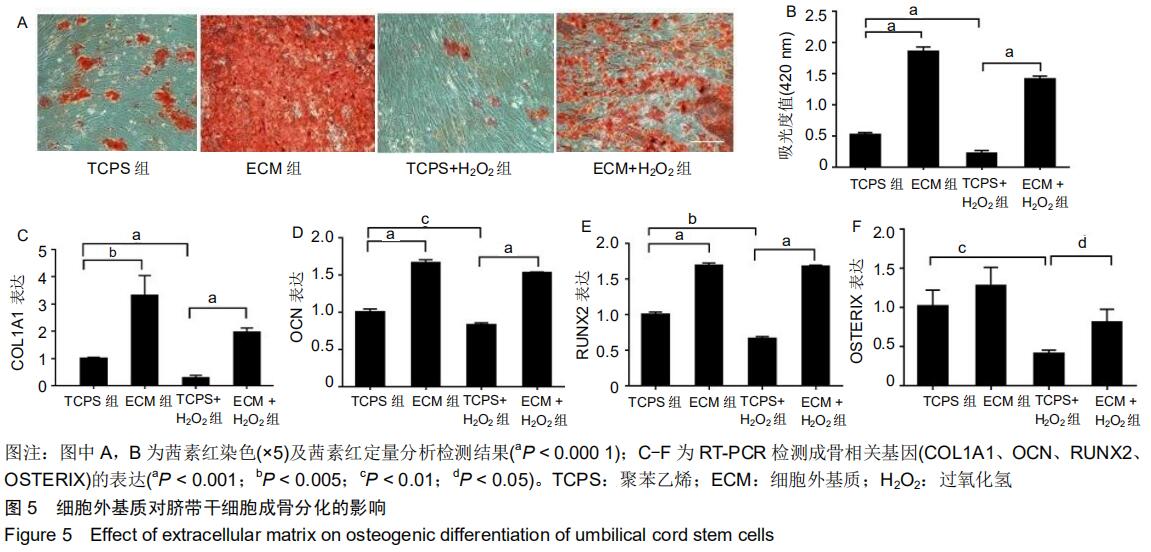
|
[1] OREFFO RO, COOPER C, MASON C, et al. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1(2):169-178.
[2] BARRY F, MURPHY M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9(10):584-594.
[3] KONG L, ZHENG LZ, QIN L, et al. Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Translat. 2017;9:89-103.
[4] PAPPA KI, ANAGNOU NP. Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regen Med. 2009;4(3): 423-433.
[5] JANCZEWSKI AM, WOJTKIEWICZ J, MALINOWSKA E, et al. Can Youthful Mesenchymal Stem Cells from Wharton's Jelly Bring a Breath of Fresh Air for COPD? Int J Mol Sci. 2017;18(11): E2449.
[6] HSIEH JY, FU YS, CHANG SJ, et al. Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton's jelly of umbilical cord. Stem Cells Dev. 2010;19(12):1895-1910.
[7] MITCHELL KE, WEISS ML, MITCHELL BM, et al. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21(1):50-60.
[8] SCHIEBER M, CHANDEL NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453-462.
[9] MINIERI V, SAVIOZZI S, GAMBAROTTA G, et al. Persistent DNA damage-induced premature senescence alters the functional features of human bone marrow mesenchymal stem cells. J Cell Mol Med. 2015; 19(4):734-743.
[10] ZELKO IN, MARIANI TJ, FOLZ RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33(3):337-349.
[11] HE F, LIU X, XIONG K, et al. Extracellular matrix modulates the biological effects of melatonin in mesenchymal stem cells. J Endocrinol. 2014;223(2):167-180.
[12] BHAT A, BOYADJIEV SA, SENDERS CW, et al. Differential growth factor adsorption to calvarial osteoblast-secreted extracellular matrices instructs osteoblastic behavior. PLoS One. 2011;6(10):e25990.
[13] PEI M, ZHANG Y, LI J, et al. Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis. Stem Cells Dev. 2013;22(6):889-900.
[14] LIU-BRYAN R, TERKELTAUB R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11(1): 35-44.
[15] COURTIAL L, PICCO V, GROVER R, et al. The c-Jun N-terminal kinase prevents oxidative stress induced by UV and thermal stresses in corals and human cells. Sci Rep. 2017;7:45713.
[16] KAMATA H, HONDA S, MAEDA S, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120(5):649-661.
[17] SKÓRKOWSKA-TELICHOWSKA K, KULMA A, GĘBAROWSKI T, et al. V79 Fibroblasts Are Protected Against Reactive Oxygen Species by Flax Fabric. Appl Biochem Biotechnol. 2018;184(1):366-385.
[18] HAN MJ, KIM BY, YOON SO, et al. Cell proliferation induced by reactive oxygen species is mediated via mitogen-activated protein kinase in Chinese hamster lung fibroblast (V79) cells. Mol Cells. 2003; 15(1): 94-101.
[19] YANG C, YOU D, HUANG J, et al. Effects of AURKA-mediated degradation of SOD2 on mitochondrial dysfunction and cartilage homeostasis in osteoarthritis. J Cell Physiol. 2019;234(10): 17727-17738.
[20] OHTA Y, KONGO M, KISHIKAWA T. Effect of melatonin on changes in hepatic antioxidant enzyme activities in rats treated with alpha-naphthylisothiocyanate. J Pineal Res. 2001;31(4):370-377.
[21] JIANG T, XU G, WANG Q, et al. In vitro expansion impaired the stemness of early passage mesenchymal stem cells for treatment of cartilage defects. Cell Death Dis. 2017;8(6):e2851.
[22] PEI M, HE F, KISH VL. Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A. 2011;17(23-24): 3067-3076.
[23] HARVESTINE JN, SAIZ JR AM, LEACH JK. Cell-secreted extracellular matrix influences cellular composition sequestered from unprocessed bone marrow aspirate for osteogenic grafts. Biomater Sci. 2019;7(5): 2091-2101.
[24] LI J, HANSEN KC, ZHANG Y, et al. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials. 2014; 35(2):642-653.
[25] YANG Y, MELZER C, BUCAN V, et al. Conditioned umbilical cord tissue provides a natural three-dimensional storage compartment as in vitro stem cell niche for human mesenchymal stroma/stem cells. Stem Cell Res Ther. 2016;7:28.
[26] QU H, LIU X, NI Y, et al. Laminin 411 acts as a potent inducer of umbilical cord mesenchymal stem cell differentiation into insulin-producing cells. J Transl Med. 2014;12:135.
[27] LAI Y, SUN Y, SKINNER CM, et al. Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev. 2010;19(7):1095-1107.
[28] LIN H, YANG G, TAN J, et al. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials. 2012;33(18): 4480-4489.
[29] WEN JH, VINCENT LG, FUHRMANN A, et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater. 2014;13(10):979-987.
[30] ZHOU L, CHEN X, LIU T, et al. Melatonin reverses H2 O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J Pineal Res. 2015;59(2):190-205.
[31] BYON CH, JAVED A, DAI Q, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283(22):15319-15327.
[32] LEE DH, LIM BS, LEE YK, et al. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 2006;22(1): 39-46.
[33] YOON SO, YUN CH, CHUNG AS. Dose effect of oxidative stress on signal transduction in aging. Mech Ageing Dev. 2002;123(12): 1597-1604.
|