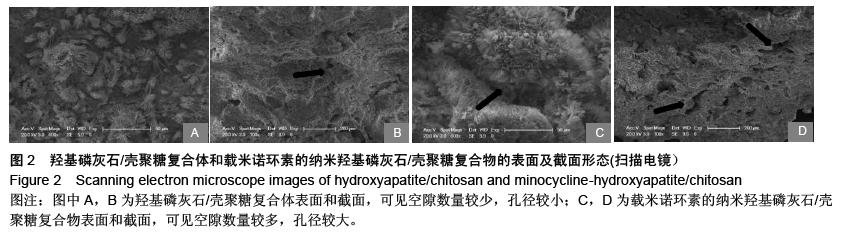
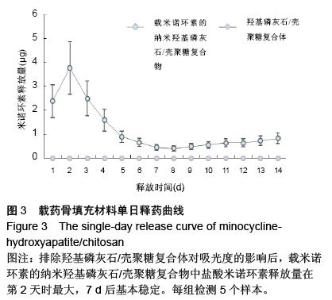
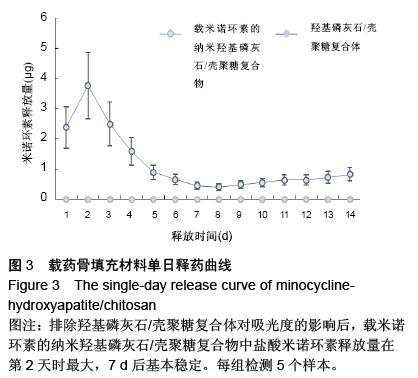
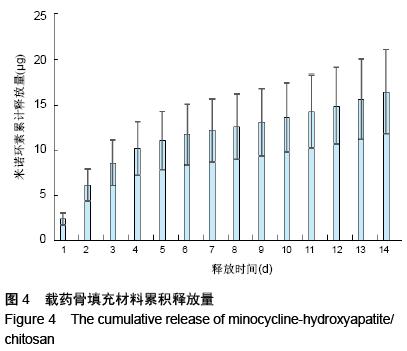
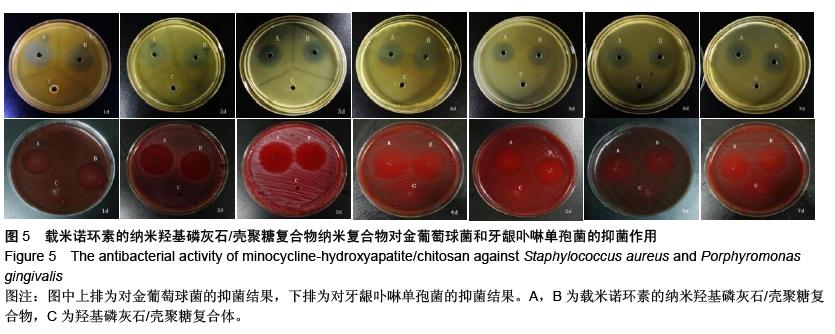
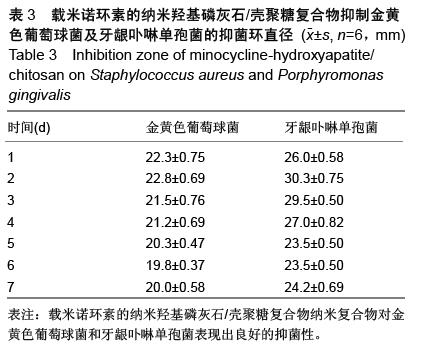
| [1] 王金成,薛白,葛葵葵,等.搭载溶葡萄球菌酶羟基磷灰石/壳聚糖复合材料的可控释放[J].药学学报,2014,49(9): 1331-1339.
[2] Li Y, Liu T, Zheng J, et al. Glutaraldehyde-crosslinked chitosan/hydroxyapatite bone repair scaffold and its application as drug carrier for icariin. J Appl Polym Sci. 2013;130(3):1539-1547.
[3] 任韵清,杨森,张学军.米诺环素应用新进展[J].中华皮肤科杂志, 2005,38(12):777-778.
[4] Tran RT, Thevenot P, Zhang Y, et al. Scaffold Sheet Design Strategy for Soft Tissue Engineering. Nat Mater. 2010;3(2):1375-1389.
[5] 麻健丰,刘劲松,张大风,等.3种牙科铸造金属模拟唾液浸泡后粗糙度的变化[J].上海口腔医学, 2007,16(3):307-310.
[6] 莫志江,危华玲.利用SPSS软件估算药物体外释放参数[J].时珍国医国药,2007,18(6):1419-1420.
[7] Dou XC, Zhu XP, Zhou J, et al. Minocycline-released hydroxyapatite-gelatin nanocomposite and its cytocompatibility in vitro. Biomed Mater. 2011;6(2): 025002.
[8] Javed S, Kohli K. Local delivery of minocycline hydrochloride: a therapeutic paradigm in periodontal diseases. Curr Drug Deliv. 2010;7(5):398-406.
[9] Muthukuru M, Sun J. Doxycycline counteracts bone morphogenic protein 2-induced osteogenic mediators. J Periodontol. 2013;84(5):656-665.
[10] Madhumathi K, Sampath Kumar TS. Regenerative potential and anti-bacterial activity of tetracycline loaded apatitic nanocarriers for the treatment of periodontitis. Biomed Mater. 2014;9(3):035002.
[11] Zhang R, Ma PX. Poly(alpha-hydroxyl acids)/ hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J Biomed Mater Res. 1999;44(4):446-455.
[12] Chang BS, Lee CK, Hong KS, et al. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials. 2000;21(12):1291-1298.
[13] De Souza R, Zahedi P, Allen CJ, et al. Biocompatibility of injectable chitosan-phospholipid implant systems. Biomaterials. 2009;30(23-24):3818-3824.
[14] Dai T, Tanaka M, Huang YY, et al. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther. 2011; 9(7):857-879.
[15] Freier T, Koh HS, Kazazian K, et al. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials. 2005;26(29):5872-5878.
[16] Zhang L, Tang P, Zhang W, et al. Effect of chitosan as a dispersant on collagen-hydroxyapatite composite matrices. Tissue Eng Part C Methods. 2010;16(1): 71-79.
[17] 宁思敏,王东,孙海钰,等.大鼠成骨细胞与壳聚糖/羟基磷灰石复合支架降解产物的生物相容性[J].中国组织工程研究, 2014,18(12):1846-1851.
[18] Sutha S, Kavitha K, Karunakaran G, et al. In-vitro bioactivity, biocorrosion and antibacterial activity of silicon integrated hydroxyapatite/chitosan composite coating on 316 L stainless steel implants. Mater Sci Eng C Mater Biol Appl. 2013;33(7):4046-4054.
[19] 赵宏霞.载药壳聚糖/羟基磷灰石复合材料在骨组织工程中的研究进展[J].功能材料,2014,(13):13006-13012.
[20] 袁华,陈宁,吕晓迎,等.天然羟基磷灰石-壳聚糖复合材料修复骨缺损的实验研究[J].口腔医学,2005,25(2):65-67.
[21] 许勇,朱立新,田京,等.可注射性纳米羟基磷灰石?壳聚糖复合材料修复骨缺损的实验研究[J].中国临床解剖学杂志, 2009,27(6):712-715.
[22] Yasaei M, Zamanian A, Moztarzadeh F, et al. Characteristics improvement of calcium hydroxide dental cement by hydroxyapatite nanoparticles. Part 1: formulation and microstructure. Biotechnol Appl Biochem. 2013;60(5):502-509.
[23] O'Connor BC, Newman HN, Wilson M. Susceptibility and resistance of plaque bacteria to minocycline. J Periodontol. 1990;61(4):228-233.
[24] Nakashima K, Suido H, Eguchi T, et al. Antibiotic therapy in periodontal disease. 1. Selection of antibiotics. Nihon Shishubyo Gakkai Kaishi. 1987; 29(2): 463-471.
[25] Gao C, Cai Y, Kong X, et al. Development and characterization of injectable chitosan-basedhydrogels containing dexamethasone/rhBMP-2 loadedhydroxyapatite nanoparticless. Mater Lett. 2013;93(1):312-315.
[26] 张达,屠冠军,韩亚新,等.米诺环素微环境对体内外骨髓间充质干细胞的影响[J].中国医科大学学报, 2013,42(1): 1-3. |