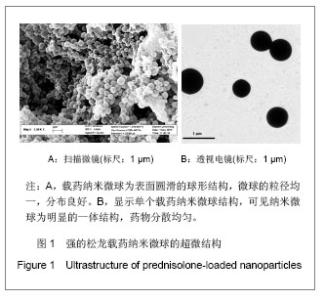
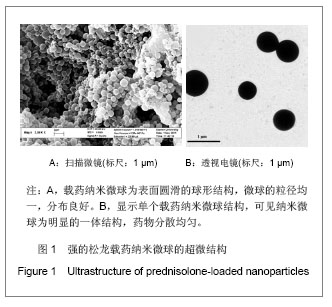
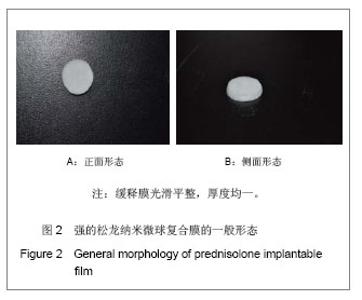
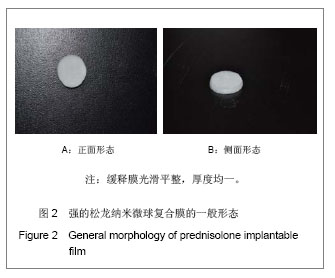
Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (51): 8849-8855.doi: 10.3969/j.issn.2095-4344.2013.51.011
Previous Articles Next Articles
Biosafety of prednisolone implantable film
Tang Yu-sen, Li Qiang, Qi Peng, Lian Ke-jian
- Department of Orthopedics, the 175 Hospital of PLA, Zhangzhou 363000, Fujian Province, China
-
Online:2013-12-17Published:2013-12-17 -
Contact:Lian Ke-jian, Chief physician, Department of Orthopedics, the 175 Hospital of PLA, Zhangzhou 363000, Fujian Province, China 306108982@qq.com -
About author:Tang Yu-sen, Attending physician, Department of Orthopedics, the 175 Hospital of PLA, Zhangzhou 363000, Fujian Province, China 306108982@qq.com -
Supported by:the Science and Technology Foundation of Zhangzhou City, No. Z2010086*
CLC Number:
Cite this article
Tang Yu-sen, Li Qiang, Qi Peng, Lian Ke-jian. Biosafety of prednisolone implantable film[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(51): 8849-8855.
share this article
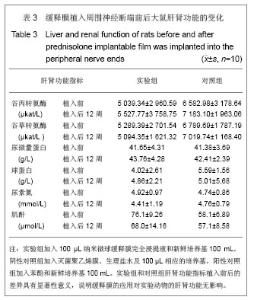
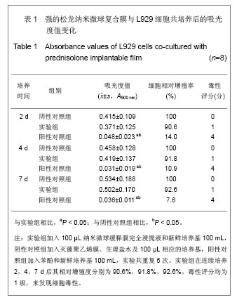
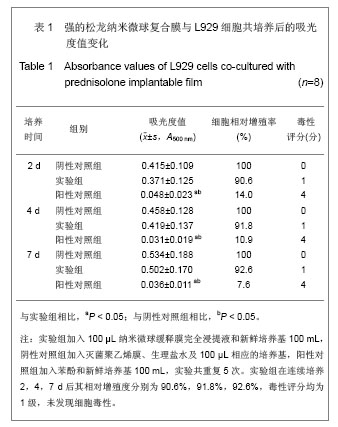
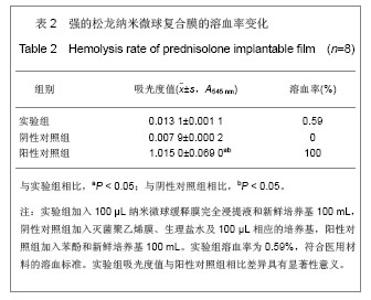
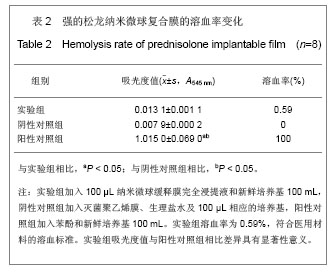
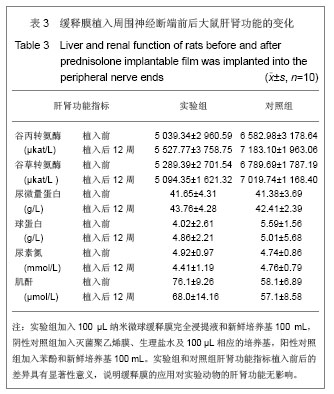
2.5 急性全身毒性实验结果 将缓释膜的浸取液注入SD大鼠体内后的24,48,72 h 内所有实验动物均未发现行为学的异常,精神状态及活动正常,未见大鼠出现呼吸困难、畏寒、厌食、腹泻及体质量下降等症状。 2.6 慢性全身毒性反应结果 见表3。植入后12周实验组与对照组大鼠均未出现明显的行为学的异常,未见自噬及生活特性的改变,精神状态及活动正常,未见大鼠出现呼吸困难、畏寒、厌食、腹泻及体质量下降等症状。两组植入前、植入后12周肝肾功能无异常。 2.7 生物相容性评价 上述各项实验结果中可看出,在细胞毒性实验、溶血实验、急性毒性实验、慢性毒性实验中该缓释膜均显示出了较好的生物学相容性,实验基本达到了预期的结果,但是在作者对慢性毒性实验动物的取材中发现,在缓释膜植入动物体内的初期,大鼠局部仍然有一定程度的炎症反应出现,且炎症反应的程度与缓释膜载药量相关,但长期观察发现后期炎症反应逐渐消退,因此在缓释膜的进一步研究中笔者将对缓释膜载药量与局部炎症反应方面进一步实验。"
| [1]Cui F, Shi K, Zhang L, et al. Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: preparation, in vitro characterization and in vivo evaluation. J Control Release. 2006;114(2):242-250.[2]Deumens R, Bozkurt A, Meek MF, Marcus MA, Joosten EA, Weis J, et al. Repairing injured peripheral nerves: bridging the gap. Prog Neurobiol. 2010;92(3):245-276.[3]IJpma FF, Van De Graaf RC, Meek MF. The early history of tubulation in nerve repair. J Hand Surg Eur Vol. 2008;33(5): 581-586.[4]Yates JM, Smith KG, Robinson PP. The effect of triamcinolone hexacetonide on the spontaneous and mechanically-induced ectopicdischarge following lingual nerve injury in the ferret. Pain. 2004;111(3):261-269.[5]Azuma C, Tohyama H, Nakamura H, et al. Antibody neutralization of TGF-beta enhances the deterioration of collagen fascicles in a tissue-cultured tendon matrix with ex vivo fibroblast infiltration. J Biomech. 2007;40(10):2184-2190.[6]赵永青,苏衍萍,韩凤岳.甲基强的松龙对急性脊髓损伤神经元保护作用的实验研究[J].中华神经外科杂志,2005,21(6):367-370. [7]张海英,李玉珍.糖皮质激素类药物的药理特性及合理应用[J].临床药物治疗杂志,2004,2(3):36-42.[8]王晓波.药物运释系统[M].北京:中国医药科技出版社,2007.[9]Takezawa T, T akeuchi T, Nitani A, et al. Collagen vitr igel membrane useful for parac-rine assays in vit ro and drug delivery systems in vivo. J Biotechnol. 2007;131(1):76-83.[10]Bellamkonda RV. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials. 2006; 27(19):3515-3518.[11]Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82(4):163-201.[12]The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30. [13]Fournier C, Hecquet B, Bouffard R, et al. Experimental studies and preliminary clinical trial of vinorelbine-loaded polymeric bioresorbable implants for the local treatment of solid tumors. Cancer Res. 1991;51(19):5384-5391.[14]Wang J, Ng CW, Win KY, et al. Release of paclitaxel from polylactide-co-glycolide (PLGA) microparticles and discs under irradiation. J Microencapsulation. 2003;20(3):317-327.[15]Shameem M, Lee H, Burton K, et al. Effect of γ-irradiation on peptide-containing hydrophilic poly (d,l-lactide-co-glycolide) microspheres. J Pharm Sci Technol. 1999;53(6):309-315.[16]林晓艳,唐敏,张兴栋.γ射线低温辐照对胶原膜体外稳定性和细胞相容性的影响[J].生物医学工程学杂志,2006,23(4):822-825.[17]杨飞,杨吟野,李训虎,等.γ射线辐照对壳聚糖薄膜的改性研究[J].透析与人工器官,2003,2(14):14-19.[18]Ruiter GC, Onyeneho IA, Liang ET, et al. Methods for in vitro characterization of multichannel nerve tubes. J Biomed Mater Res A. 2008;84(3):643-651.[19]Graham WP 3rd, Davis TS, Miller SH, et al. Efficacy of triamcinolone acetonide following neurorrhaphy-an electroneuromy ographic evaluation. Ann Plast Surg. 1982; 9(3):230-237.[20]Nel A, Xia T, Mädler L, et al. Toxic potential ofmateri-als at the nanolevel. Science. 2006;311(5761):622-627.[21]陆兵,袁本利,徐国杰,等.纳曲酮植入剂不同动物体内的组织相容性和生物可降解性[J].中国药学杂志,2000,35(6):391-393.[22]Lee SH, Park M, Park CG, et al. Microchip for sustained drug delivery by diffusion through microchannels. AAPS Pharm Sci Tech. 2012;13(1):211-217.[23]Ni S, Lin K, Chang J, et al. β-CaSiO3/β-Ca3(PO4)2 composite materials for hard tissue repair:In vitro studies. J Biomed Mater Res A. 2008;85(1):72-82.[24]Sclapp M, Friess W. Collagen/PLGA microparticle composites for local controlled delivery of gentamicin. J Pharm Sci. 2003; 92(11):2145-2151.[25]Jeong B, Bae YH, Kim SK, et al. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J Control Release. 2000;63(1-2):155-163.[26]Bini TB, Gao S, Xu X, et al. Peripheral nerve re-generation by microbraided poly (L-lactide-co-glycol-ide)biodegradable polymer fibers. J Biomed Mater Res A. 2004;68(2):286-295.[27]Chang CJ, Hsu. The effect of high outflow permeability in asymmetric poly(dl-lactic acid-co-glycolic acid) conduits for peripheralnerve regeneration. Biomaterials. 2006;27(7): 1035-1042.[28]叶震海,顾立强.雪旺细胞和聚乳酸材料的体外相容性研究[J].中华创伤骨科杂志,2000,2(4):306-307.[29]Liu P, Sun Y, Wang Q, et al. Intracellular trafficking and cellular uptake mechanism of mPEG-PLGA-PLL and mPEG-PLGA-PLL-Gal nanoparticles for targeted delivery to hepatomas. Biomaterials. 2014;35(2):760-770.[30]张文元,杨亚冬,张科技,等.蚕丝-聚乳酸-聚羟基乙酸纤维混合编织绳状支架的生物学性能[J].中华实验外科杂志,2013,30(5): 931-933. |
| [1] | Tang Mengmeng, Chen Hechun, Xie Hongchen, Zhang Yu, Tan Xiaoshuang, Sun Yixuan, Huang Yina. Histocompatibility of poly(L-lactide-co-ε-caprolactone)/cross-linked polyvinylpyrrolidone ureteral stent grafted into the rat bladder [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(4): 583-588. |
| [2] | Guo Enhui, Xu Zitong, Liang Yize, Zhou Liang, Lu Zhaoxiang, You Liang, Xia Yujun. Properties of a novel photocrosslinked fish collagen peptide-hyaluronic acid hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(28): 4518-4525. |
| [3] | Cheng Lei, Jin Jian, Hu Jingguo, Lu Yusong. Inflammatory reaction and lactic acid concentration after implantation of polylactic acid rib nail versus pure titanium embracing fixator in animals#br# [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(16): 2567-2571. |
| [4] | Zhou Yanxing1, Peng Xinsheng2, Hou Gan1, Li Jiangbin1, Zhang Hua1, Zhou Zhikun2, Zhou Yanfang3 . Inhibitory effect of capsaicin on fibroblast proliferation and its molecular mechanism [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(7): 1018-1022. |
| [5] | Cheng Jian, Zhang Jun, Guan Jie, Zeng Junkai, Zhao Xin, Xie Youzhuan. Silver nanoparticle-doped tricalcium phosphate: in vitro and in vivo toxicity in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(6): 917-923. |
| [6] | Tian Hongju, Chen Zhongqing. Poly(lactid-co-glycolide) nanoparticles loaded with ropivacaine: preparation and in vivo release in animals [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(6): 924-929. |
| [7] | Wu Bo, Li Xiangkui, Wang Hua, Wen Zhiyuan. Sustained-release properties and histocompatibility of ropivacaine-coated polyethylene glycol/polylactic acid microspheres implanted around the sciatic nerve [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(34): 5486-5491. |
| [8] | Xu Xiaoling, Pan Wangping, Lü Xiaojun, Zhang Ju, Hu Yuanhua, He Kaiyong. Immunotoxicity of absorbable silk fibroin biofilm on rats [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(14): 2190-2195. |
| [9] | Lu Yangzhou, Li Shao, Jiang Hua, Li Wei, Gao Yi. Pre-clinical acute toxicity of an immortalized human hepatocyte cell line HepZJ [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(13): 2067-2074. |
| [10] | Jing Ya-jun 1, Zhang Lei1, Zhang Shao-qun1, Liao Li-qing1, Yuan Shi-guo2, Li Yi-kai1. Pathological changes of the skeletal muscle after local loosening therapies [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(4): 535-541. |
| [11] | Ge Wenjia, Ma Xiaorong, Ouyang Tianxiang . Aminolevulinic acid photodynamic therapy can significantly inhibit the growth of endothelial cells and induce cell apoptosis [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(36): 5840-5845. |
| [12] | Liu Tian-jia, Gu Shuo, Zhou Lu, Zhong Jia-fei, Yuan Guang-yin. Safety and effectiveness of a biodegradable magnesium alloy fixation system in animals [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(30): 4876-4881. |
| [13] | Liu Meng-yuan, Jing Miao-lei, Guan Jing, Huang Shu-jie, Yang Jian, Li Zhi-hong. Preparation of N-hexane chitosan and its cytotoxicity and coagulation property [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(22): 3520-3526. |
| [14] | Wang Xiao, Xu Hong-guang, Xiao Liang, Liu Chen, Jin Zhong-xing, Shen Yang. Porcine decellular endplate cartilage matrix: biocompatibility assessment and establishment of a cytotoxicity evaluation system [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(22): 3539-3544. |
| [15] | Liu Xue-jian, Liu Shi-chen, Sun Bai-chuan, Zhang Kai-hong, Meng Hao-ye, Wang Yu Huang Shao-dai, Lu Chang-feng, Wang Chong, Yu Wen, Jing Xiao-guang, Zhao Yue, Yang Jian-hua, Peng Jiang . Preparation and characterization of laser microporous acellular osteochondral scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(18): 2836-2842. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||