Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (1): 78-92.doi: 10.12307/2026.510
Previous Articles Next Articles
Effects of human umbilical cord blood platelet-rich plasma, mononuclear cells, and mesenchymal stem cells in repairing thin endometrium in rats
Mu Yanli1, Hu Anchun1, Xu Wenchi1, Chen Panpan1, Chen hao2, Zhao Shuyun2, Huang Guanyou2, Chen Xiaojuan3
- 1Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 2Department of Reproductive Center, 3Department of Hyperbaric Oxygen, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China
-
Received:2024-10-31Accepted:2024-12-31Online:2026-01-08Published:2025-06-19 -
Contact:Zhao Shuyun, MD, Chief physician, Department of Reproductive Center, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Mu Yanli, Master candidate, Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
Supported by:Guizhou Provincial Health Commission Science and Technology Fund, No. gzwkj2013-1-101 (to HGY); Guizhou Provincial Health Commission Science and Technology Fund, No. gzwkj2022-415 (to CXJ); Guizhou Provincial Health Commission Science and Technology Fund, No. g2wkj2024-439 (to HAC)
CLC Number:
Cite this article
Mu Yanli, Hu Anchun, Xu Wenchi, Chen Panpan, Chen hao, Zhao Shuyun, Huang Guanyou, Chen Xiaojuan. Effects of human umbilical cord blood platelet-rich plasma, mononuclear cells, and mesenchymal stem cells in repairing thin endometrium in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 78-92.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
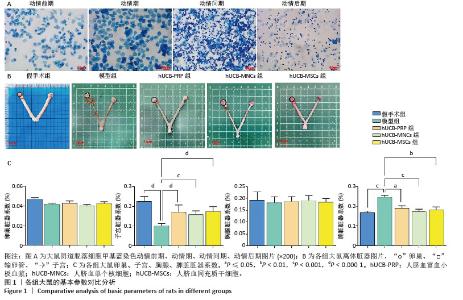
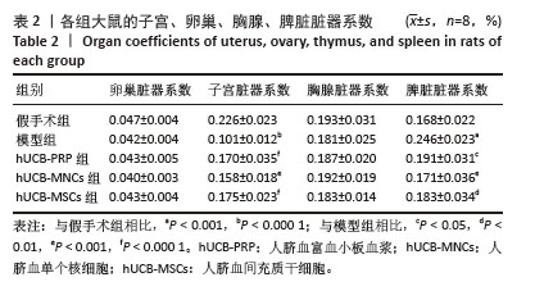
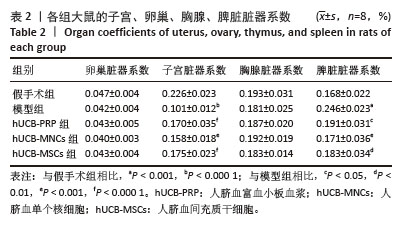
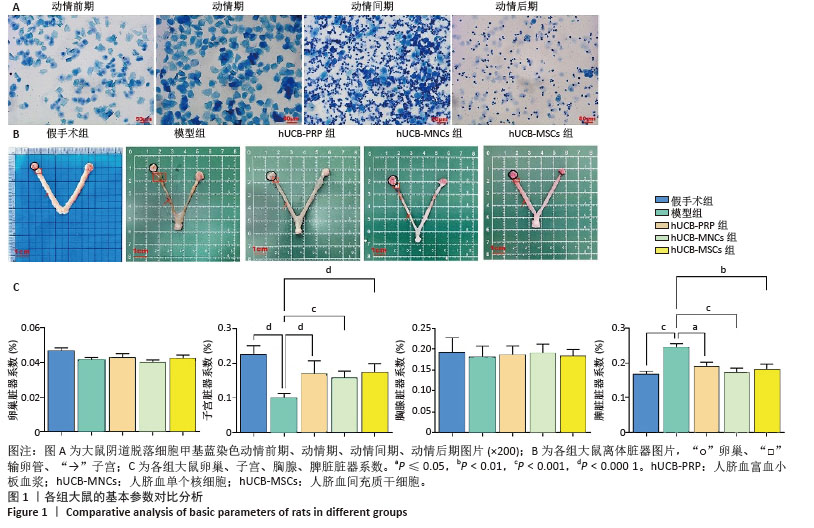
2.1 实验动物数量分析 购进67只雌性大鼠,经大鼠动情周期测定,舍弃7只动情周期紊乱大鼠,60只大鼠进入结果分析。 2.2 各组大鼠基本参数对比 阴道脱落细胞涂片检查确认SD大鼠的动情周期,主要表现为动情前期(P):全部是有核上皮细胞,偶有少量角化细胞;动情期(E):全部是无核角化细胞或间有少量上皮细胞;动情间期(M):白细胞、角化细胞、有核上皮细胞均有;动情后期(DI):大量白细胞及少量上皮细胞和黏液,见图1A。 动情周期正常大鼠入组,再灌注后第3个正常动情周期的动情间期取大鼠子宫组织。假手术组子宫组织大小正常,形态规则,呈粉红色,组织质地均匀、富有弹性,切面观内膜厚度适中,血管分布丰富且均匀,无明显肿块与淤血坏死。模型组子宫组织萎缩,形态不规则,呈棕黄色,表面凹凸不平,组织僵硬,切面观内膜变薄,血管分布稀疏,大部分血管扩张显露出淤血,部分可见输卵管积水,由此说明通过体积分数95%乙醇维持灌注子宫,可造成实质性损伤。与假手术组相比,模型组子宫和脾脏脏器系数具有显著差异(P < 0.001)。hUCB-PRP组子宫较模型组有所改善,形态较不规则,粗细不均,部分区域呈现出水肿,颜色略显苍白,切面观子宫内膜厚度和血管分布中等,无明显淤血坏死区域。hUCB-MNCs组子宫组织大小欠佳,形态欠规则,颜色趋于正常,表面欠光滑,切面观内膜厚度和血管分布有所改善,可见少量淤血坏死区域。 hUCB-MSCs组子宫组织大小接近正常,形态较规则,呈淡粉色,组织表面光滑,有一定弹性,切面观内膜厚度趋向正常,血管分布丰富,未见明显淤血坏死区域,见图1B。此外,与模型组相比,脐血衍生物各组在子宫和脾脏脏器系数上呈现出差异性的改善(P < 0.01),但脐血衍生物组间差异不显著。与模型组相比,各组卵巢、脾脏脏器系数尚未发现有统计学意义,见表2,图1C。 "
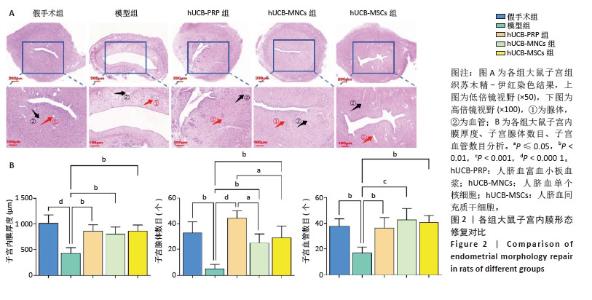
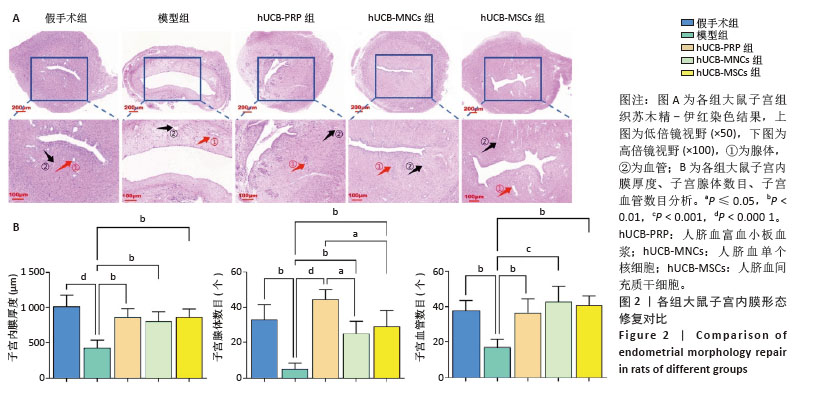
2.3 各组大鼠子宫内膜形态 人脐血衍生物对受损后的大鼠子宫内膜具有不同程度的修复作用,主要表现在增加子宫内膜腺体与血管的生成,促进生长因子、激素调节因子、营养物质和黏液的分泌,支持胚胎着床和早期妊娠。实验研究结果显示,假手术组子宫内膜显示正常的多层上皮细胞,结构完整,表面由柱状上皮覆盖,细胞核形态规则,细胞质均匀;基质细胞分布均匀,无明显炎症反应;腺体呈现良好的圆形或卵形结构,开口清晰,腺上皮细胞呈柱状;血管结构清晰,排列有序,无明显增生或退化。与假手术组相比,模型组子宫内膜厚度减少,上皮细胞层变薄,细胞核形态不规则,部分细胞凋亡;基质细胞减少,可见炎性细胞浸润;腺体数量明显减少,部分腺体形态不规则,腺腔萎缩,腺上皮细胞减少;血管结构紊乱,部分血管壁增厚,内皮细胞损伤。经过hUCB-PRP、hUCB-MSCs、hUCB-MNCs宫腔灌注治疗后,3个治疗组的子宫内膜厚度均有所增加(P < 0.01),上皮细胞层修复良好,细胞核形态逐渐规则;基质细胞增加,纤维化减轻,炎性细胞浸润减少,见图2A。组间比较显示,与hUCB-PRP组相比,hUCB-MSCs组和hUCB-MNCs组腺体数量均降低(P < 0.05)。除此之外,子宫内膜厚度、血管数目尚未发现显著差异,见图2B。 "
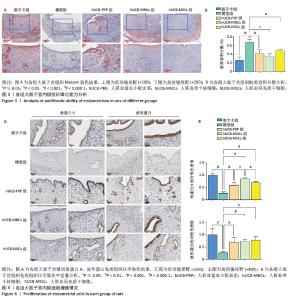
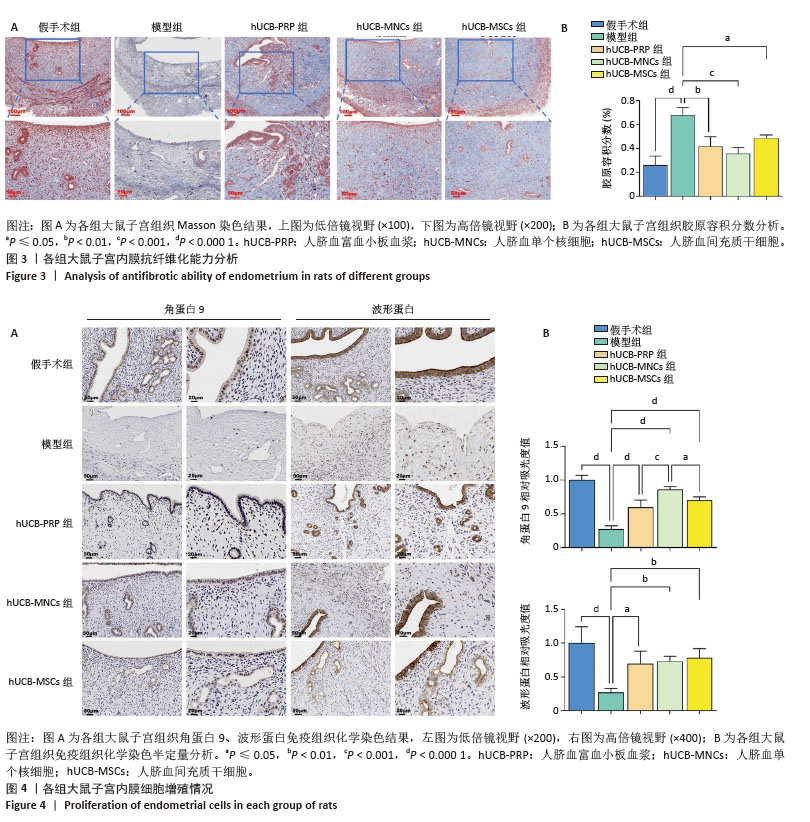
2.4 各组大鼠子宫内膜抗纤维化能力 Masson染色后大鼠子宫组织胶原纤维呈蓝色,肌纤维呈现红色。在正常的大鼠子宫内膜中,肌纤维主要位于子宫肌层中,胶原纤维主要位于大鼠子宫内膜的间质以及最外层的包膜中。当子宫的抗纤维化能力降低后,子宫组织中细胞外基质的过度积累,导致组织结构和功能改变,进而通过基质微环境、子宫内膜容受性、血管生成、免疫调节等方面对胚胎着床产生影响[54-57]。 假手术组胶原纤维少量均匀分布在子宫内膜间质,无明显过度增生或缺失;细胞核呈红色,基质细胞分布正常,无明显纤维化迹象。与假手术组相比,模型组胶原纤维过度增生,分布不均匀;细胞核红色变淡,基质细胞明显减少。 hUCB-PRP组与hUCB-MSCs组胶原纤维增生减少,分布欠均匀,主要分布于子宫内膜基质层;细胞核红色较浓,基质细胞增多。hUCB-MNCs组胶原纤维分布均匀,纤维化显著减轻;细胞核红色浓密,基质细胞数量显著增加,见图3A。以胶原容积分数来量化各组子宫组织纤维化程度,结果显示:与假手术组相比,模型组出现显著纤维化,差异有显著性意义(P < 0.000 1)。与模型组相比,hUCB-PRP、hUCB-MSCs与hUCB-MNCs宫腔灌注治疗后,纤维化水平明显改善,以hUCB-MNCs组最为明显,差异有显著性意义(P < 0.05),见图3B。 2.5 各组大鼠子宫内膜细胞增殖情况 角蛋白在子宫内膜中的表达主要局限于上皮细胞和腺上皮细胞中,是细胞骨架的重要组成成分,主要功能是维持子宫上皮细胞的组织连续性和完整性。而波形蛋白的表达局限于子宫内膜的间质,是间质细胞骨架的重要组成成分,不仅可以维持细胞结构稳定,还参与细胞有丝分裂、增殖分化及胞内信号转导[53]。免疫组化结果显示,与假手术组相比,模型组角蛋白9、波形蛋白表达水平显著降低(P < 0.000 1)。与模型组相比,hUCB-PRP、hUCB-MNCs与hUCB-MSCs宫腔灌注治疗后,角蛋白9、波形蛋白表达水平均有一定程度的上升(P < 0.01),见图4A。进一步组间比较,与hUCB-MNCs组相比,hUCB-PRP组与hUCB-MSCs组角蛋白9表达水平均降低(P < 0.05)。波形蛋白表达水平组间比较尚未发现统计学差异,见图4B。 "
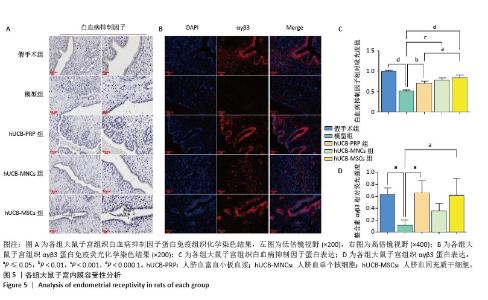
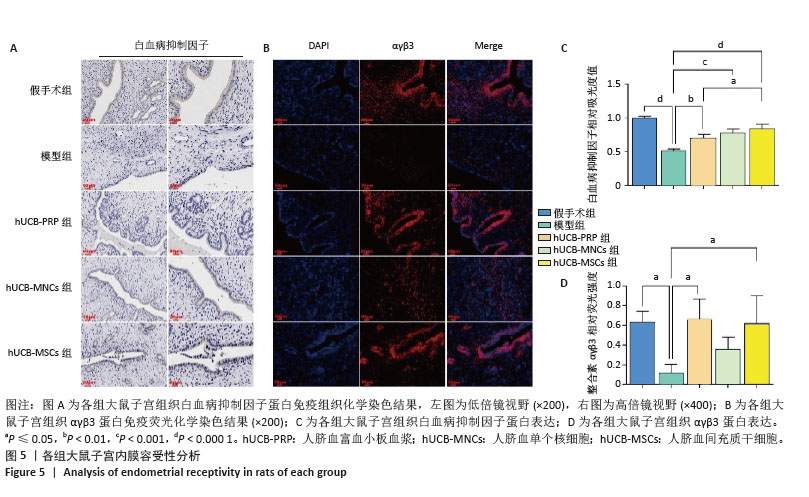
2.6 各组大鼠子宫内膜容受性 整合素αγβ3是一种细胞黏附分子,广泛表达于子宫内膜上皮细胞和基质细胞中,特别是在子宫内膜的种植窗口期表达最高。白血病抑制因子作为一种多效性细胞因子,与整合素αγβ3具有协同作用,故整合素αγβ3蛋白和白血病抑制因子蛋白表达升高有利于胚胎的着床与植入。与假手术组相比,模型组整合素αγβ3蛋白和白血病抑制因子蛋白表达水平显著下降(P < 0.05)。经人脐血衍生物治疗后,与模型组相比,除hUCB-MNCs组整合素αγβ3蛋白表达尚未发现显著升高外,其余组别的整合素αγβ3蛋白和白血病抑制因子蛋白表达水平均有所升高,差异有显著性意义(P < 0.05),见图5A-D。 "
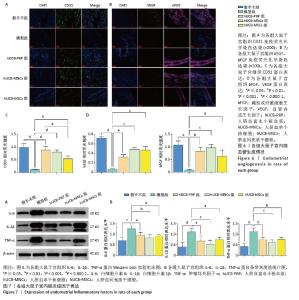
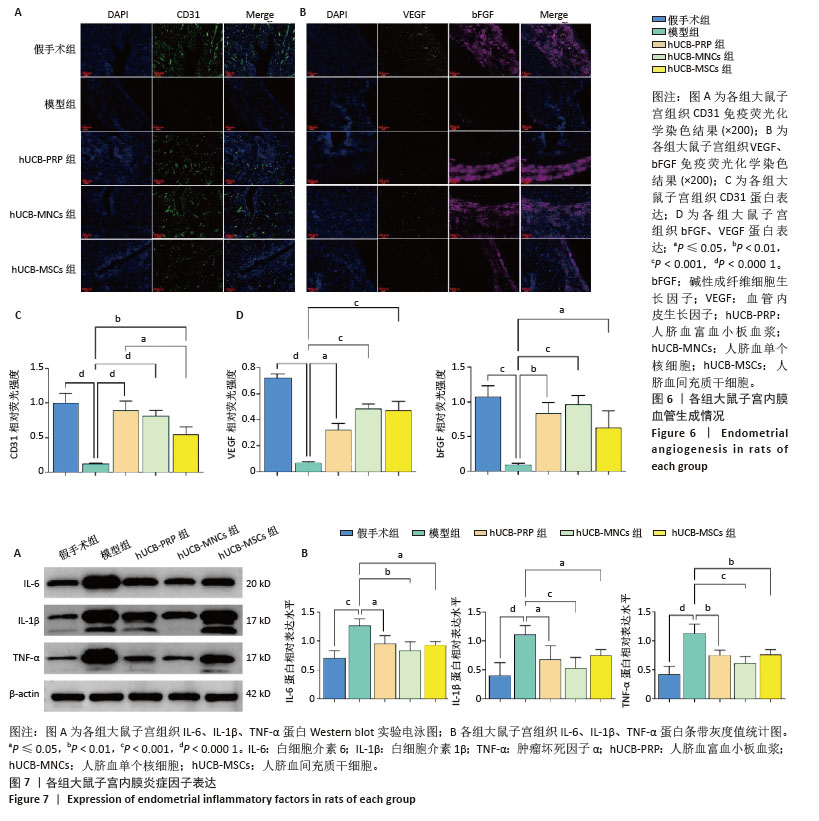
2.7 各组大鼠子宫内膜血管生成情况 血管内皮生长因子和碱性成纤维细胞生长因子是重要的血管生成因子,能够促进血管内皮细胞的增殖和迁移。此外,作为存在于内皮细胞表面的一种黏附分子,血小板内皮细胞黏附分子(CD31)也参与内皮细胞之间的黏附及新生血管的形成。在受损的子宫内膜中,血管生成是修复的关键机制,为再生组织提供必要的氧气和营养物质。与假手术组相比,模型组血管内皮生长因子、碱性成纤维细胞生长因子和CD31蛋白表达水平均有所下降,而经过人脐血衍生物治疗后,血管内皮生长因子、碱性成纤维细胞生长因子和CD31蛋白表达水平均有回升,差异有显著性意义(P < 0.05),见图6A,B。其中血管内皮生长因子蛋白表达水平以hUCB-MNCs和hUCB-MSCs组回升较为明显(P < 0.001),而CD31蛋白表达水平从hUCB-PRP组到hUCB-MNCs组再到hUCB-MSCs组逐渐降低,但与模型组相比,均有所升高,差异有显著性意义(P < 0.05)。碱性成纤维细胞生长因子则以hUCB-MNCs组增加最显著(P < 0.001),见图6C,D。 2.8 各组大鼠子宫内膜炎症反应 在子宫内膜的修复过程中,炎症反应扮演着重要角色。白细胞介素6、白细胞介素1β和肿瘤坏死因子α等炎症因子的适度表达有助于清除损伤并促进组织修复,但过度的炎症反应可能干扰修复过程,导致不良后果。因此,通过人脐血衍生物来平衡炎症反应,能够有效修复子宫内膜。与假手术组相比,模型组白细胞介素6、白细胞介素1β和肿瘤坏死因子α蛋白表达水平均显著升高(P < 0.001)。经人脐血衍生物灌注治疗后,白细胞介素6、白细胞介素1β和肿瘤坏死因子α蛋白表达水平均降低(P < 0.05),其中以hUCB-MNCs组抑制炎症与免疫调控作用最为显著(P < 0.01)。白细胞介素6、白细胞介素1β和肿瘤坏死因子α蛋白表达水平在3个治疗组间未见差异(P > 0.05),见图7A,B。 "
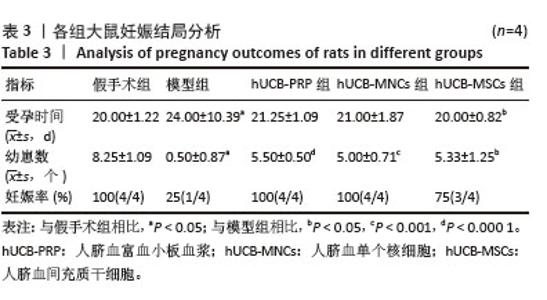
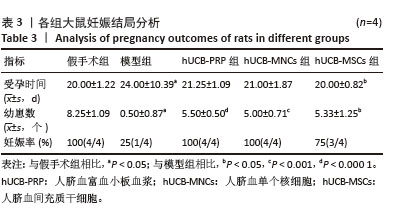
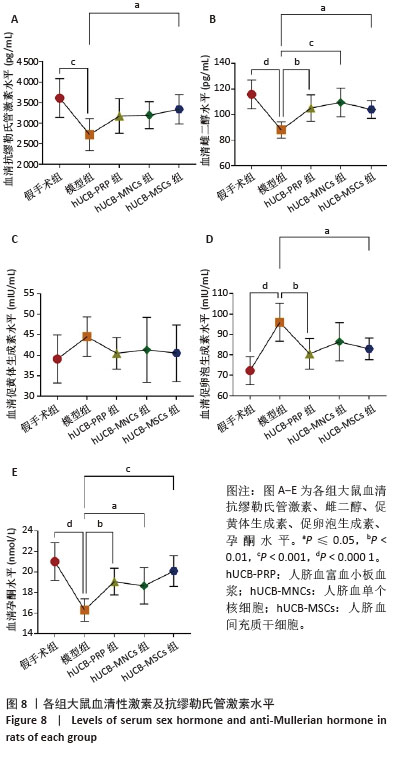
2.9 各组大鼠血清性激素及抗缪勒氏管激素水平 大鼠血清中性激素及抗缪勒氏管激素水平的变化与子宫内膜的修复过程密切相关,这些激素的平衡状态有助于促进子宫内膜的再生和修复,而异常的激素水平可能影响修复效果,甚至导致不良后果。因此,监测这些激素水平可帮助评估和调节子宫内膜的修复过程。与假手术组相比,模型组血清雌二醇、促黄体生成素、促卵泡生成素、孕酮及抗缪勒氏管激素水平均向不良方向增加,除促黄体生成素尚未发现显著变化外,其余指标差异均有显著性意义(P < 0.001)。经过人脐血衍生物治疗后,与模型组相比,hUCB-MSCs组抗缪勒氏管激素水平增加,hUCB-PRP组和hUCB-MSCs组促卵泡生成素水平稍有降低,hUCB-PRP组、hUCB-MNCs组和hUCB-MSCs组雌二醇与孕酮水平均升高,差异有显著性意义(P < 0.05),见图8。 "
| [1] SAAD-NAGUIB MH, KENFACK Y, SHERMAN LS, et al. Impaired receptivity of thin endometrium: therapeutic potential of mesenchymal stem cells. Front Endocrinol (Lausanne). 2024;14:1268990. [2] LIU X, QIAN C, JIANG X, et al. Efficacy of platelet-rich plasma in the treatment of thin endometrium: a meta-analysis of randomized controlled trials. BMC Pregnancy Childbirth. 2024;24(1):567. [3] WEISS NS, VAN VLIET MN, LIMPENS J, et al. Endometrial thickness in women undergoing IUI with ovarian stimulation. How thick is too thin? A systematic review and meta-analysis. Hum Reprod. 2017;32(5): 1009-1018. [4] LIU KE, HARTMAN M, HARTMAN A, et al. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. 2018;33(10):1883-1888. [5] KASIUS A, SMIT JG, TORRANCE HL, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(4):530-541. [6] MOUHAYAR Y, SHARARA FI. G-CSF and stem cell therapy for the treatment of refractory thin lining in assisted reproductive technology. J Assist Reprod Genet. 2017;34(7):831-837. [7] MAO X, ZHANG J, CAI R, et al. Therapeutic role of granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with persistent thin endometrium: A prospective and randomized study. Int J Gynaecol Obstet. 2020;150(2):194-199. [8] MAKRIGIANNAKIS A, MAKRYGIANNAKIS F, VREKOUSSIS T. Approaches to Improve Endometrial Receptivity in Case of Repeated Implantation Failures. Front Cell Dev Biol. 2021;9:613277. [9] LV H, ZHAO G, JIANG P, et al. Deciphering the endometrial niche of human thin endometrium at single-cell resolution. Proc Natl Acad Sci U S A. 2022;119(8):e2115912119. [10] SHU J, LIU X, LI R. Editorial: Impaired receptivity of thin endometrium: the mechanism, hormone intervention and strategies. Front Endocrinol (Lausanne). 2024;15:1432284. [11] WANG Y, TANG Z, TENG X. New advances in the treatment of thin endometrium. Front Endocrinol (Lausanne). 2024;15:1269382. [12] ZHAO J, ZHANG Q, WANG Y, et al. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod Sci. 2015;22(2):181-188. [13] HAO SN, XIA LJ, XI J, et al. Electroacupuncture combined with bone marrow mesenchymal stem cell transplantation promotes repair of thin endometrium by regulating SDF-1/CXCR4 signaling. Zhen Ci Yan Jiu. 2023;48(9):870-880. [14] ARIKAN G, TURAN V, KUREKEKEN M, et al. Autologous bone marrow-derived nucleated cell (aBMNC) transplantation improves endometrial function in patients with refractory Asherman’s syndrome or with thin and dysfunctional endometrium. J Assist Reprod Genet. 2023;40(5):1163-1171. [15] YE MX, YU L, WANG SF, et al. Efficacy of gamma-irradiated adipose-derived stem cells for treatment of thin endometrium in rats. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37(5):575-580. [16] SUDOMA I, PYLYP L, KREMENSKA Y, et al. Application of autologous adipose-derived stem cells for thin endometrium treatment in patients with failed ART programs. J Stem Cell Ther Transplant. 2019;3(1): 001-008. [17] YOTSUMOTO F, YOSHIKAWA K, HIRAKAWA T, et al. Safety and Potential Effect of Intrauterine Infusion of Autologous Adipose Tissue-Derived Regenerative Cells in Patients With Implantation Failure: A Pilot Study. Cureus. 2024;16(3):e57220. [18] ZHAO M, CHI F, ZHANG T, et al. Human menstrual blood-derived mesenchymal stem cells regulation of the EGF/Ras p21 pathway as a potential therapeutic target for thin endometrium. Ann Transl Med. 2021;9(18):1476. [19] WANG H, CHEN K, ZONG L, et al. MALAT1/miR-7-5p/TCF4 Axis Regulating Menstrual Blood Mesenchymal Stem Cells Improve Thin Endometrium Fertility by the Wnt Signaling Pathway. Cell Transplant. 2024;33:9636897241259552. [20] ZHANG Y, SHI L, LIN X, et al. Unresponsive thin endometrium caused by Asherman syndrome treated with umbilical cord mesenchymal stem cells on collagen scaffolds: a pilot study. Stem Cell Res Ther. 2021;12(1):420. [21] ZHOU S, LEI Y, WANG P, et al. Human Umbilical Cord Mesenchymal Stem Cells Encapsulated with Pluronic F-127 Enhance the Regeneration and Angiogenesis of Thin Endometrium in Rat via Local IL-1β Stimulation. Stem Cells Int. 2022;2022:7819234. [22] SAPOZHAK IM, GUBAR ОS, RODNICHENKO AE, et al. Application of autologous endometrial mesenchymal stromal/stem cells increases thin endometrium receptivity: a case report. J Med Case Rep. 2020; 14(1):190. [23] RODRÍGUEZ-EGUREN A, BUENO-FERNANDEZ C, GÓMEZ-ÁLVAREZ M, et al. Evolution of biotechnological advances and regenerative therapies for endometrial disorders: a systematic review. Hum Reprod Update. 2024;30(5):584-613. [24] AGARWAL M, METTLER L, JAIN S, et al. Management of a Thin Endometrium by Hysteroscopic Instillation of Platelet-Rich Plasma Into The Endomyometrial Junction: A Pilot Study. J Clin Med. 2020; 9(9):2795. [25] YUAN G, YU C, DU X, et al. Injectable GelMA Hydrogel Microspheres with Sustained Release of Platelet-Rich Plasma for the Treatment of Thin Endometrium. Small. 2024;20(47):e2403890. [26] WANG X, LIU L, MOU S, et al. Investigation of platelet-rich plasma in increasing proliferation and migration of endometrial mesenchymal stem cells and improving pregnancy outcome of patients with thin endometrium. J Cell Biochem. 2019;120(5):7403-7411. [27] TABEEVA G, SILACHEV D, VISHNYAKOVA P, et al. The Therapeutic Potential of Multipotent Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Endometrial Regeneration. Int J Mol Sci. 2023;24(11):9431. [28] ZHOU Y, LI Q, YOU S, et al. Efficacy of Mesenchymal Stem Cell-Derived Extracellular Vesicles in the Animal Model of Female Reproductive Diseases: A Meta-Analysis. Stem Cell Rev Rep. 2023;19(7):2299-2310. [29] GIBSON OO, MAKARCHUK O, RIMARCHUK M, et al. Role of vascular growth factors as a regulator of angiogenesis processes at the stage of implantation potential formation in women with uterine factor infertility. Health of Woman. 2019;6(142):27-33. [30] WANG S, LIU T, NAN N, et al. Exosomes from Human Umbilical Cord Mesenchymal Stem Cells Facilitates Injured Endometrial Restoring in Early Repair Period through miR-202-3p Mediating Formation of ECM. Stem Cell Rev Rep. 2023;19(6):1954-1964. [31] WANG S, SHI C, CAI X, et al. Human Acellular Amniotic Matrix with Previously Seeded Umbilical Cord Mesenchymal Stem Cells Restores Endometrial Function in a Rat Model of Injury. Mediators Inflamm. 2021;2021:5573594. [32] SONG L, ZHANG Q, ZHU S, et al. Granulocyte Colony-Stimulating Factor Combined With Transcutaneous Electrical Acupoint Stimulation in Treatment of Unresponsive Thin Endometrium in Frozen Embryo Transfer Cycles. Front Reprod Health. 2021;3:647336. [33] PARAJULI R, JAIN V, GAINDER S, et al. An interventional study on the effect of intrauterine granulocyte-colony stimulating factor instillation on thin or damaged endometrium in women undergoing assisted reproduction. Fertil Steril. 2024;122(4):e425. [34] CHEN J, HUANG F, FU J, et al. Hyperbaric oxygen therapy: a possible choice for patients with resistant thin endometrium during frozen embryo transfer treatments. Reprod Biol Endocrinol. 2023;21(1):80. [35] RODRÍGUEZ-EGUREN A, GÓMEZ-ÁLVAREZ M, FRANCÉS-HERRERO E, et al. Human Umbilical Cord-Based Therapeutics: Stem Cells and Blood Derivatives for Female Reproductive Medicine. Int J Mol Sci. 2022;23(24):15942. [36] RODRÍGUEZ-EGUREN A, DE MIGUEL-GÓMEZ L, FRANCÉS-HERRERO E, et al. Human umbilical cord platelet-rich plasma to treat endometrial pathologies: methodology, composition and pre-clinical models. Hum Reprod Open. 2022;2023(1):hoac053. [37] CECERSKA-HERYĆ E, GOSZKA M, SERWIN N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. [38] LEE OK, KUO TK, CHEN WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669-1675. [39] XI Y, YUE G, GAO S, et al. Human umbilical cord blood mononuclear cells transplantation for perinatal brain injury. Stem Cell Res Ther. 2022;13(1):458. [40] BAZANOVICH SA, RYABOV SI, ZVYAGINTSEVA MA, et al. Evaluation of the Effectiveness of Systemic Therapy of Spinal Cord Injury of Moderate Severity with Human Umbilical Cord Placental Blood Mononuclear Cells Using Indicators of Dispersion of Articular Angles in the Swimming Test. Bull Exp Biol Med. 2023;174(6):784-789. [41] SHULMAN I, OGURCOV S, KOSTENNIKOV A, et al. Application of Autologous Peripheral Blood Mononuclear Cells into the Area of Spinal Cord Injury in a Subacute Period: A Feasibility Study in Pigs. Biology (Basel). 2021;10(2):87. [42] LI J, JIANG Y, XUE W, et al. Effects of transplantation of umbilical cord blood mononuclear cells into the scrotum on sexual function in elderly mice. Regen Med. 2023;18(9):695-706. [43] CAI X, LI Y, GAO F, et al. Therapeutic effect and study of human umbilical cord blood mononuclear cells in patients with ischaemic bowel disease. Sci Rep. 2024;14(1):6121. [44] CHEN G, YUE A, YU H, et al. Mesenchymal Stem Cells and Mononuclear Cells From Cord Blood: Cotransplantation Provides a Better Effect in Treating Myocardial Infarction. Stem Cells Transl Med. 2016;5(3):350-357. [45] ZHANG L, LI Y, DONG YC, et al. Transplantation of umbilical cord-derived mesenchymal stem cells promotes the recovery of thin endometrium in rats. Sci Rep. 2022;12(1):412. [46] 冯海艳,邓春雷,张怡君,等.薄型子宫内膜大鼠95%乙醇造模法的实验研究[J].湖北医药学院学报,2024,43(5):490-493+458. [47] WANG J, QIN W, ZHONG Y, et al. Injectable collagen hydrogel combines human umbilical cord mesenchymal stem cells to promote endometrial regeneration in rats with thin endometrium. Int J Biol Macromol. 2024;254(Pt 1):127591. [48] XIA L, MENG Q, XI J, et al. The synergistic effect of electroacupuncture and bone mesenchymal stem cell transplantation on repairing thin endometrial injury in rats. Stem Cell Res Ther. 2019;10(1):244. [49] GUO Q, CHANG Y, LI J, et al. Regenerative Effects of Locally or Intra-Arterially Administered BMSCs on the Thin Endometrium. Front Bioeng Biotechnol. 2022;10:735465. [50] LIN Y, DONG S, YE X, et al. Synergistic regenerative therapy of thin endometrium by human placenta-derived mesenchymal stem cells encapsulated within hyaluronic acid hydrogels. Stem Cell Res Ther. 2022;13(1):66. [51] LI J, HUANG B, DONG L, et al. WJ-MSCs intervention may relieve intrauterine adhesions in female rats via TGF-β1-mediated Rho/ROCK signaling inhibition. Mol Med Rep. 2021;23(1):15. [52] XIE Y, TIAN Z, QI Q, et al. The therapeutic effects and underlying mechanisms of the intrauterine perfusion of granulocyte colony-stimulating factor on a thin-endometrium rat model. Life Sci. 2020; 260:118439. [53] 刘若熹.人脐带间充质干细胞修复大鼠薄型子宫内膜的研究[D].济南:山东大学,2020. [54] GARGETT CE, YE L. Endometrial reconstruction from stem cells. Fertil Steril. 2012;98(1):11-20. [55] LIU F, HU S, WANG S, et al. Cell and biomaterial-based approaches to uterus regeneration. Regen Biomater. 2019;6(3):141-148. [56] ZHANG Z, LI S, DENG J, et al. Aspirin inhibits endometrial fibrosis by suppressing the TGF β1 Smad2/Smad3 pathway in intrauterine adhesions. Int J Mol Med. 2020;45(5):1351-1360. [57] AI Y, CHEN M, LIU J, et al. lncRNA TUG1 promotes endometrial fibrosis and inflammation by sponging miR-590-5p to regulate Fasl in intrauterine adhesions. Int Immunopharmacol. 2020;86:106703. [58] WORLD HEALTH ORGANIZATION. WHO Team Sexual and Reproductive Health and Research Infertility Prevalence Estimates 1990-2021. Geneva: World Health Organization, 2024. [59] CAIAFFA V, IPPOLITO F, ABATE A, et al. Allogenic platelet concentrates from umbilical cord blood for knee osteoarthritis: preliminary results. Med Glas (Zenica). 2021;18(1):260-266. [60] YANG Q, HUANG J, LIU Y, et al. Human Umbilical Cord Mesenchymal Stem Cells Promote Anti-Inflammation and Angiogenesis by Targeting Macrophages in a Rat Uterine Scar Model. Stem Cell Rev Rep. 2024;20(6):1555-1568. [61] SHIN SY, CHUNG N, SHIN JE, et al. Angiogenic factor-driven improvement of refractory thin endometrium with autologous platelet-rich plasma intrauterine infusion in frozen embryo transfer cycles. Front Endocrinol (Lausanne). 2024;15:1431453. [62] JING Z, YI Y, XI H, et al. Therapeutic Effects of VEGF Gene-Transfected BMSCs Transplantation on Thin Endometrium in the Rat Model. Stem Cells Int. 2018;2018:3069741. [63] LV H, XU R, XIE X, et al. Injectable, degradable, and mechanically adaptive hydrogel induced by L-serine and allyl-functionalized chitosan with platelet-rich plasma for treating intrauterine adhesions. Acta Biomater. 2024;184:144-155. [64] KIM MK, YOON JA, YOON SY, et al. Human Platelet-Rich Plasma Facilitates Angiogenesis to Restore Impaired Uterine Environments with Asherman’s Syndrome for Embryo Implantation and Following Pregnancy in Mice. Cells. 2022;11(9):1549. [65] ZADEHMODARRES S, SALEHPOUR S, SAHARKHIZ N, et al. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod. 2017;21(1):54-56. [66] SFAKIANOUDIS K, SIMOPOULOU M, NITSOS N, et al. Successful Implantation and Live Birth Following Autologous Platelet-rich Plasma Treatment for a Patient with Recurrent Implantation Failure and Chronic Endometritis. In Vivo. 2019;33(2):515-521. [67] MOLINA A, SÁNCHEZ J, SÁNCHEZ W, et al. Platelet-rich plasma as an adjuvant in the endometrial preparation of patients with refractory endometrium. JBRA Assist Reprod. 2018;22(1):42-48. [68] ZHANG S, LI P, YUAN Z, et al. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res Ther. 2019;10(1):61. [69] TANDULWADKAR S, MISHRA S, GUPTA S. Successful Application of Combined Autologous Bone Marrow-Derived Stem Cells and Platelet-Rich Plasma in a Case of Severe Asherman Syndrome and Subsequent in vitro Fertilization Conception. J Hum Reprod Sci. 2021;14(4):446-449. [70] LI J, LI X, DING J, et al. Analysis of pregnancy outcomes in patients with recurrent implantation failure complicated with chronic endometritis. Front Cell Dev Biol. 2023;11:1088586. [71] CASTELLANO JM, MOSHER KI, ABBEY RJ, et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017;544(7651):488-492. [72] EHRHART J, SANBERG PR, GARBUZOVA-DAVIS S. Plasma derived from human umbilical cord blood: Potential cell-additive or cell-substitute therapeutic for neurodegenerative diseases. J Cell Mol Med. 2018;22(12):6157-6166. [73] MURPHY MB, BLASHKI D, BUCHANAN RM, et al. Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials. 2012;33(21):5308-5316. [74] LEHALLIER B, GATE D, SCHAUM N, et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019;25(12): 1843-1850. [75] NAGAMURA-INOUE T, HE H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195-202. [76] ALANAZI A, ALASSIRI M, JAWDAT D, et al. Mesenchymal stem cell therapy: A review of clinical trials for multiple sclerosis. Regen Ther. 2022;21:201-209. [77] CHENG H, LIU X, HUA R, et al. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253. [78] DING DC, CHANG YH, SHYU WC, et al. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339-347. [79] HE X, WANG Q, ZHAO Y, et al. Effect of Intramyocardial Grafting Collagen Scaffold With Mesenchymal Stromal Cells in Patients With Chronic Ischemic Heart Disease: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(9):e2016236. [80] BARTOLUCCI J, VERDUGO FJ, GONZÁLEZ PL, et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121(10):1192-1204. [81] ÖZMERT E, ARSLAN U. Management of retinitis pigmentosa by Wharton’s jelly-derived mesenchymal stem cells: prospective analysis of 1-year results. Stem Cell Res Ther. 2020;11(1):353. [82] ZANG L, LI Y, HAO H, et al. Efficacy and safety of umbilical cord-derived mesenchymal stem cells in Chinese adults with type 2 diabetes: a single-center, double-blinded, randomized, placebo-controlled phase II trial. Stem Cell Res Ther. 2022;13(1):180. [83] HUANG J, LI Q, YUAN X, et al. Intrauterine infusion of clinically graded human umbilical cord-derived mesenchymal stem cells for the treatment of poor healing after uterine injury: a phase I clinical trial. Stem Cell Res Ther. 2022;13(1):85. [84] XU Y, HU J, LV Q, et al. Endometrium-derived mesenchymal stem cells suppress progression of endometrial cancer via the DKK1-Wnt/β-catenin signaling pathway. Stem Cell Res Ther. 2023;14(1):159. [85] BENVENUTO F, VOCI A, CARMINATI E, et al. Human mesenchymal stem cells target adhesion molecules and receptors involved in T cell extravasation. Stem Cell Res Ther. 2015;6:245. [86] JIAO Y, LI XY, LIU J. A New Approach to Cerebral Palsy Treatment: Discussion of the Effective Components of Umbilical Cord Blood and its Mechanisms of Action. Cell Transplant. 2019;28(5):497-509. [87] AHN JY, HONG YH, KIM KC, et al. Effect of Human Peripheral Blood Mononuclear Cells on Mouse Endometrial Cell Proliferation: A Potential Therapeutics for Endometrial Regeneration. Gynecol Obstet Invest. 2022;87(2):105-115. [88] ZHAO G, CAO Y, ZHU X, et al. Transplantation of collagen scaffold with autologous bone marrow mononuclear cells promotes functional endometrium reconstruction via downregulating ΔNp63 expression in Asherman’s syndrome. Sci China Life Sci. 2017;60(4):404-416. [89] RALLAPALLI S, GUHATHAKURTA S, NARAYAN S, et al. Generation of clinical-grade red blood cells from human umbilical cord blood mononuclear cells. Cell Tissue Res. 2019;375(2):437-449. [90] ZHANG J, ZHAI H, YU P, et al. Human Umbilical Cord Blood Mononuclear Cells Ameliorate CCl4-Induced Acute Liver Injury in Mice via Inhibiting Inflammatory Responses and Upregulating Peripheral Interleukin-22. Front Pharmacol. 2022; 13:924464. [91] RAMLI Y, ALWAHDY AS, KURNIAWAN M, et al. Intra-arterial Transplantation of Human Umbilical Cord Blood Mononuclear Cells in Sub-acute Ischemic Stroke Increases VEGF Expression in Rats. J Stem Cells Regen Med. 2018;14(2):69-79. [92] GATINA DZ, GAZIZOV IM, ZHURAVLEVA MN, et al. Induction of Angiogenesis by Genetically Modified Human Umbilical Cord Blood Mononuclear Cells. Int J Mol Sci. 2023;24(5):4396. [93] CHO KH, CHOI JI, KIM JO, et al. Therapeutic mechanism of cord blood mononuclear cells via the IL-8-mediated angiogenic pathway in neonatal hypoxic-ischaemic brain injury. Sci Rep. 2020;10(1):4446. [94] EFENDIEVA Z, VISHNYAKOVA P, APOLIKHINA I, et al. Hysteroscopic injections of autologous endometrial cells and platelet-rich plasma in patients with thin endometrium: a pilot randomized study. Sci Rep. 2023;13(1):945. [95] YUAN G, LI D, DU X, et al. Effects of platelet-rich fibrin on human endometrial stromal cells behavior in comparison to platelet-rich plasma. Front Cell Dev Biol. 2024;12:1445928. [96] XIE Q, LIU R, JIANG J, et al. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment. Stem Cell Res Ther. 2020;11(1):519. [97] BABA K, YAMAZAKI Y, SONE Y, et al. An in vitro long-term study of cryopreserved umbilical cord blood-derived platelet-rich plasma containing growth factors-PDGF-BB, TGF-β, and VEGF. J Craniomaxillofac Surg. 2019;47(4):668-675. [98] ZHU D, CHENG K. Cardiac Cell Therapy for Heart Repair: Should the Cells Be Left Out. Cells. 2021;10(3):641. [99] CHEN K, WANG H, ZHAO X, et al. A Novel Method to Repair Thin Endometrium and Restore Fertility Based on Menstruation-Derived Stem Cell. Reprod Sci. 2024;31(6):1662-1673. [100] LIN X, FANG Y, MI X, et al. Intrauterine injection of bioengineered hydrogel loaded exosomes derived from HUCM stem cells and spermidine prominently augments the pregnancy rate in thin endometrium rats. Regen Ther. 2024;27:63-72. [101] XIN L, LIN X, PAN Y, et al. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019;92:160-171. [102] XU L, DING L, WANG L, et al. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res Ther. 2017;8(1):84. [103] SHUAI Q, LIANG Y, XU X, et al. Sodium alginate hydrogel integrated with type III collagen and mesenchymal stem cell to promote endometrium regeneration and fertility restoration. Int J Biol Macromol. 2023; 253(Pt 6):127314. [104] KHARBIKAR BN, MOHINDRA P, DESAI TA. Biomaterials to enhance stem cell transplantation. Cell Stem Cell. 2022;29(5):692-721. [105] HE X, HONG W, YANG J, et al. Spontaneous apoptosis of cells in therapeutic stem cell preparation exert immunomodulatory effects through release of phosphatidylserine. Signal Transduct Target Ther. 2021;6(1):270. [106] NÉMETH K, LEELAHAVANICHKUL A, YUEN PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42-49. |
| [1] | He Longcai, Song Wenxue, Ming Jiang, Chen Guangtang, Wang Junhao, Liao Yidong, Cui Junshuan, Xu Kaya. An experimental method for simultaneous extraction and culture of primary cortical neurons and microglial cells from SD rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1395-1400. |
| [2] | Zhuo Qiuyan, Jiang Qun, Xia Si, Lu Shiying, Liu Yandi, Dai Mei. Bone marrow hematopoiesis in rats with myelodysplastic syndrome: action mechanism of Huosui Formula in intervening immune checkpoints [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7735-7742. |
| [3] | Zhong Min, Wang Cheng, Fan Zhenhai, Li Linyan, Yu Limei. Effect and mechanism of perinatal mesenchymal stem cells and their combination with hydrogels in treatment of intrauterine adhesions [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6792-6799. |
| [4] | Liang Roujun, Zhan Lifen, Zeng Xuejiu, Ding Qiangsheng, Luo Xiaojing, Zhuo Yue, Ai Kun, Deng Shifeng, Xu Ming, Zhang Hong. Effect of the number of times to urinate on the modeling rate of neurogenic bladder model in rats after complete spinal cord transection [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3840-3847. |
| [5] | Wen Zhejia, Lyu Fang. Promotion of endometrial cell proliferation evaluated by platelet-rich plasma based on microfluidic chips [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2091-2096. |
| [6] | Ma Yanxia , Yang Yanwei , Ma Yuhang , Li Di , Wang Xiaoyan , Zou Mingming , Wei Shanwen. Lnx1 expression in cortical neurons of rats with traumatic brain injury and mechanisms involved in secondary brain injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 24-30. |
| [7] | Liao Yidong, Ming Jiang, Song Wenxue, Wang Zili, Zhang Yu, Liao Yifei, Xu Kaya, Yang Hua. An experimental method for simultaneously culturing primary cortical and hippocampal neurons [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 897-902. |
| [8] | Liu Xingyu, Hu Xiaofang, Xu Guangli, Wang Rui, Li Peiyao, Wang Mengyuan, Peng Li, Zhu Xiangying. Human umbilical cord mesenchymal stem cells in endometrial injury repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3921-3927. |
| [9] | Wang Wenhua, Liu Yang, Wang Qiaomin, Lü Yang, Zhao Yaru, Wang Haiping. 5-Azacytidine combined with bone morphogenetic protein 2 induces differentiation of bone marrow mesenchymal stem cells into cardiomyocyte-like cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 2958-2963. |
| [10] | Deng Liu-xia, Yu Guo-long, Ai Qi, Yuan Chun-ju. Effects of intravenous transplantation of human umbilical cord blood mononuclear cells combined with perindopril on inflammatory response of acute myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(19): 3481-3487. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||