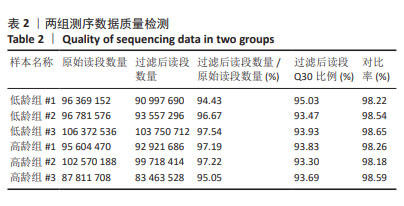
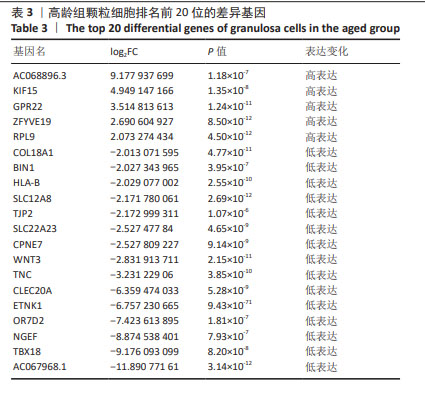
1] CECCONI S, CICCARELLI C, BARBERI M, et al. Granulosa cell-oocyte interactions. Eur J Obstet Gynecol Reprod Biol. 2004;115 Suppl 1: S19-22.
[2] GILULA NB, EPSTEIN ML, BEERS WH. Cell-to-cell communication and ovulation. A study of the cumulus-oocyte complex. J Cell Biol. 1978; 78(1):58-75.
[3] DONG JP, DAI ZH, JIANG ZX, et al. CD24: a marker of granulosa cell subpopulation and a mediator of ovulation. Cell Death Dis. 2019; 10(11):791.
[4] LI Q, MCKENZIE LJ, MATZUK MM. Revisiting oocyte-somatic cell interactions: in search of novel intrafollicular predictors and regulators of oocyte developmental competence. Mol Hum Reprod. 2008;14(12): 673-678.
[5] UYAR A, TORREALDAY S, SELI E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril. 2013;99(4):979-997.
[6] SELI E, ROBERT C, SIRARD MA. OMICS in assisted reproduction: possibilities and pitfalls. Mol Hum Reprod. 2010;16(8):513-530.
[7] HERMAN AB, TSITSIPATIS D, GOROSPE M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol Cell. 2022;82(12): 2252-2266.
[8] CHEN S, ZHOU Y, CHEN Y, et al. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884-i890.
[9] KIM D, PAGGI JM, PARK C, et al. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37(8):907-915.
[10] LIAO Y, SMYTH GK, SHI W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923-930.
[11] LUN ATL, SMYTH GK. No counts, no variance: allowing for loss of degrees of freedom when assessing biological variability from RNA-seq data. Stat Appl Genet Mol Biol. 2017;16(2):83-93.
[12] ZHOU Y, ZHOU B, PACHE L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523.
[13] SUBRAMANIAN A, TAMAYO P, MOOTHA VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545-15550.
[14] KULUS J, KRANC W, KULUS M, et al. New Gene Markers of Exosomal Regulation Are Involved in Porcine Granulosa Cell Adhesion, Migration, and Proliferation. Int J Mol Sci. 2023;24(14):11873.
[15] LATHAM KE, WIGGLESWORTH K, MCMENAMIN M, et al. Stage-dependent effects of oocytes and growth differentiation factor 9 on mouse granulosa cell development: advance programming and subsequent control of the transition from preantral secondary follicles to early antral tertiary follicles. Biol Reprod. 2004;70(5):1253-1262.
[16] DAVIS JS, FARESE RV, CLARK MR. Gonadotropin-releasing hormone (GnRH) stimulates phosphatidylinositol metabolism in rat granulosa cells: mechanism of action of GnRH. Proc Natl Acad Sci U S A. 1983; 80(7):2049-2053.
[17] MORELLI MB, BARBERI M, GAMBARDELLA A, et al. Characterization, expression, and functional activity of pituitary adenylate cyclase-activating polypeptide and its receptors in human granulosa-luteal cells. J Clin Endocrinol Metab. 2008;93(12):4924-4932.
[18] PYUN JA, KIM S, CHA DH, et al. Epistasis between IGF2R and ADAMTS19 polymorphisms associates with premature ovarian failure. Hum Reprod. 2013;28(11):3146-3154.
[19] TATONE C, AMICARELLI F, CARBONE MC, et al. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14(2): 131-142.
[20] ALVIGGI C, HUMAIDAN P, HOWLES CM, et al. Biological versus chronological ovarian age: implications for assisted reproductive technology. Reprod Biol Endocrinol. 2009;7:101.
[21] TRIPATHI A, PREMKUMAR KV, PANDEY AN, et al. Melatonin protects against clomiphene citrate-induced generation of hydrogen peroxide and morphological apoptotic changes in rat eggs. Eur J Pharmacol. 2011;667(1-3):419-424.
[22] KAWAMURA K, CHENG Y, KAWAMURA N, et al. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum Reprod. 2011;26(11):3094-3101.
[23] HSUEH AJ, KAWAMURA K, CHENG Y, et al. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36(1):1-24.
[24] GENG X, ZHAO J, HUANG J, et al. lnc-MAP3K13-7:1 Inhibits Ovarian GC Proliferation in PCOS via DNMT1 Downregulation-Mediated CDKN1A Promoter Hypomethylation. Mol Ther. 2021;29(3): 1279-1293.
[25] WU Y, XIAO H, PI J, et al. LncRNA lnc_13814 promotes the cells apoptosis in granulosa cells of duck by acting as apla-miR-145-4 sponge. Cell Cycle. 2021;20(9):927-942.
[26] YAO G, KONG Y, YANG G, et al. Lnc-GULP1-2:1 affects granulosa cell proliferation by regulating COL3A1 expression and localization. J Ovarian Res. 2021;14(1):16.
[27] CHERMUŁA B, HUTCHINGS G, KRANC W, et al. Expression Profile of New Gene Markers and Signaling Pathways Involved in Immunological Processes in Human Cumulus-Oophorus Cells. Genes (Basel). 2021; 12(9):1369.
[28] XU D, SONG S, WANG F, et al. Single-cell transcriptomic atlas of goat ovarian aging. J Anim Sci Biotechnol. 2023;14(1):151.
[29] ORIÁ RB, DE ALMEIDA JZ, MOREIRA CN, et al. Apolipoprotein E Effects on Mammalian Ovarian Steroidogenesis and Human Fertility. Trends Endocrinol Metab. 2020;31(11):872-883.
[30] ZHAO ZH, WANG XY, SCHATTEN H, et al. Single cell RNA sequencing techniques and applications in research of ovary development and related diseases. Reprod Toxicol. 2022;107:97-103.
[31] D’AURORA M, SPERDUTI S, DI EMIDIO G, et al. Inside the granulosa transcriptome. Gynecol Endocrinol. 2016;32(12):951-956.
[32] CHRONOWSKA E. High-throughput analysis of ovarian granulosa cell transcriptome. Biomed Res Int. 2014;2014:213570. |