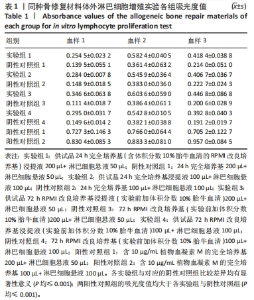
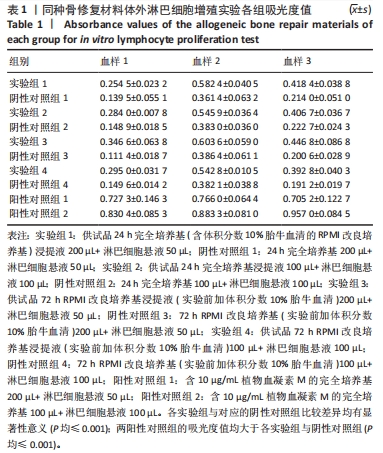
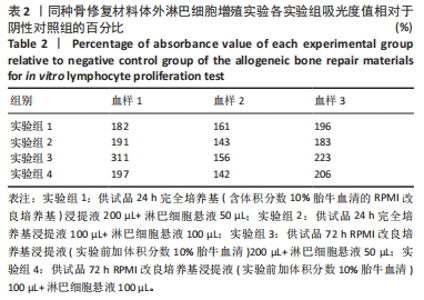
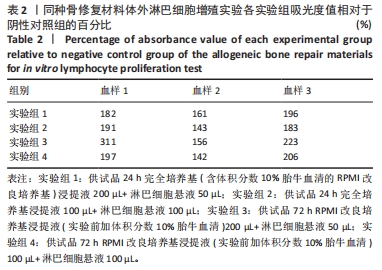
[1] RAMíREZ-MARÍN Y, ABAD-CONTRERAS DE, USTARROZ-CANO M, et al. Perfusion Decellularization of Extrahepatic Bile Duct Allows Tissue-Engineered Scaffold Generation by Preserving Matrix Architecture and Cytocompatibility. Materials (Basel). 2021;14(11):3099.
[2] MOSALA NEZHAD Z, PONCELET A, DE KERCHOVE L, et al. Small intestinal submucosa extracellular matrix (CorMatrix®) in cardiovascular surgery: a systematic review. Interact Cardiovasc Thorac Surg. 2016;22(6):839-850.
[3] NAKAMURA N, KIMURA T, KISHIDA A. Overview of the Development, Applications, and Future Perspectives of Decellularized Tissues and Organs. ACS Biomater Sci Eng. 2017;3(7):1236-1244.
[4] BADYLAK SF, GILBERT TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20(2):109-116.
[5] KASRAVI M, AHMADI A, BABAJANI A, et al. Immunogenicity of decellularized extracellular matrix scaffolds: a bottleneck in tissue engineering and regenerative medicine. Biomater Res, 2023;27(1).doi: 10.1186/s40824-023-00348-z.
[6] BOEER U, BUETTNER FF, KLINGENBERG M, et al. Immunogenicity of intensively decellularized equine carotid arteries is conferred by the extracellular matrix protein collagen type VI. PLoS One. 2014;9(8):e105964.
[7] FATANGARE A, GLÄSSNER A, SACHS B, et al. Future perspectives on in-vitro diagnosis of drug allergy by the lymphocyte transformation test. J Immunol Methods. 2021;495:113072.
[8] SACHS B, FATANGARE A, SICKMANN A, et al. Lymphocyte transformation test: History and current approaches. J Immunol Methods. 2021;493:113036.
[9] GB/T 16886.20-2015 医疗器械生物学评价 第20部分:医疗器械免疫毒理学试验 原则和方法.
[10] YY/T 1465.1-2016 医疗器械免疫原性评价方法 第1部分:体外T淋巴细胞转化试验.
[11] YY/T 0606.15-2014 组织工程医疗产品 第15部分:评价基质及支架免疫反应的试验方法:淋巴细胞增殖试验.
[12] 邵安良,穆钰峰,屈树新,等.人外周血淋巴细胞增殖试验的优化及其应用[J].药物分析杂志,2019,39(8):1354-1361.
[13] 陈亮,穆钰峰,邵安良,等.不同种属小鼠用于淋巴细胞增殖试验的比较研究[J].药物分析杂志,2019,39(8):1347-1353.
[14] 叶应妩,王毓三.全国临床检验操作规程[M].2版.南京:东南大学出版社,1997:375-385.
[15] YOSHIMURA T, KURITA C, HAYATA M, et al. Diagnosis of drug allergy by the lymphocyte stimulation test with the MTT [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay. Biol Pharm Bull. 1993;16(7):686-689.
[16] LYONS AB, PARISH CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171(1):131-137.
[17] MIYAMOTO T, MIN W, LILLEHOJ HS. Lymphocyte proliferation response during Eimeria tenella infection assessed by a new, reliable, nonradioactive colorimetric assay. Avian Dis. 2002;46(1):10-16.
[18] ALIPOUR R, ADIB M, HASHEMI-BENI B, et al. The effect of stem cell from human exfoliated deciduous teeth on T lymphocyte proliferation. Adv Biomed Res. 2014;3:202.
[19] ZHI-JUN Y, SRIRANGANATHAN N, VAUGHT T, et al. A dye-based lymphocyte proliferation assay that permits multiple immunological analyses: mRNA, cytogenetic, apoptosis, and immunophenotyping studies. J Immunol Methods. 1997;210(1):25-39.
[20] 张剑英,杨美玲,顾则娟,等.肝素与无水乙醇抑制肝癌细胞的实验研究[J].护理学杂志,2016,31(20):47-49.
[21] 张凤,张瑞华,徐薇,等.肝素抑制T细胞活化及机制研究[J].中国免疫学杂志,2009,25(3):197-201.
[22] 王宏梅,魏巍,谢涛,等.肝素对小鼠肝癌细胞Hca-F和Hca-P淋巴道转移的抑制作用[J].肿瘤防治研究,2007,34(6):435-438,475.
[23] 李文涛,王建华,尚鸣异,等.低分子肝素抑制bFGF诱导犬动脉平滑肌细胞增殖的体外实验研究[J].临床放射学杂志,2002,21(7):568-570.
[24] 张鹏,尹珺,刘毅梅.肝素和氢化可的松对血管内皮细胞和成纤维细胞的生长抑制作用[J].中国肿瘤临床,2001,28(6):19-22.
|