Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (23): 3755-3762.doi: 10.12307/2023.582
Previous Articles Next Articles
Exhaled breath condensate specimens for testing airway health during exercise
Peng Mengzhu1, Yang Xiangang2, Xing Lili3, Li Guojun3
- 1Physical Education College of Hebei Normal University, Shijiazhuang 050024, Hebei Province, China; 2Bicycle Sports Center of Hebei Sports Bureau, Qinhuangdao 066000, Hebei Province, China; 3Hebei Institute of Sports Science, Shijiazhuang 050000, Hebei Province, China
-
Received:2022-09-01Accepted:2022-10-27Online:2023-08-18Published:2023-01-16 -
Contact:Yang Xiangang, PhD, Researcher, Bicycle Sports Center of Hebei Sports Bureau, Qinhuangdao 066000, Hebei Province, China -
About author:Peng Mengzhu, Master candidate, Physical Education College of Hebei Normal University, Shijiazhuang 050024, Hebei Province, China -
Supported by:National Key Research and Development Plan for High-tech Olympics, No. 2019YFF0301602 (to YXG, XLL, LGJ [project participants])

2.1 EBC检测概述 SIDORENKO等[12]于1980年首次提出了真正意义上的EBC检测技术,用于研究肺部疾病相关的炎症因子变化。直到20世纪90年代以后,对EBC检测的研究才开始逐步增多。 EBC的收集原理是通过冷凝装置对受试者平静呼吸时的呼出气进行冷凝。通常要求受试者在收集前应使用蒸馏水仔细漱口,在正式收集过程中保持正常呼吸,避免唾液污染。通常呼出10-15 min或达到1-1.5 mL时结束,样本应置于液氮中保存等待分析[1,6]。收集装置主要包括自制和商用两大类[13-15]。常用的商用装置包括意大利的Turbodecs、美国的RTube、德国的EcoScreen,其中RTube和Turbodecss携带一次性收集器和单向非呼吸阀,可居家使用。 2.2 EBC检测运动时气道健康状况常用的生物标志物 EBC中含有的生物标志物多是水溶性,目前关于运动科学中常用的EBC的标志物大致分为炎性反应、氧化应激和其他生物标志物,前两类主要是直接参与或反映炎症反应、氧化应激的细胞因子,通过EBC检测直接提供运动后气道状态的信号;另一部分生化因子是由氧化应激或炎性因子刺激产生参与运动后气道反应。EBC检测在运动时气道健康中主要应用于通过检测出生物标志的变化特征来判断气道炎症、氧化损伤水平,提供运动人群的局部反应状态,更加准确地反映运动人群的功能状态、过度训练或赛后疲劳程度,科学地参与运动训练[1,6]。 2.2.1 常用的炎症反应类生物标志物 见表1。"

(1)pH值:EBC-pH主要由来自上皮和口腔表面的气相扩散,以及血液与肺泡间的气体交换所决定[6],被认为是反映气道酸碱平衡的炎症生物标志物,这主要是由来自口腔和气道的铵离子的质子缓冲作用调节的[24],pH值变化与中性粒细胞和嗜酸性粒细胞所介导的炎性反应有关。 (2)血栓素B2(thromboxane B2,TXB2):是花生四烯酸环氧合酶代的代谢产物,为二十烷类化合物的脂类,具有促进血管收缩和血小板凝聚的细胞因子,参与多种炎症和免疫作用,过量产生有助于慢性和急性炎症的发病机制,自身免疫和其他致病机制[25-26]。 (3)前列腺素E2(prostaglandin E2,PGE2):是一种重要的细胞生长和调节因子,主要由巨噬细胞合成,是花生四烯酸环氧合酶代谢产物,为二十碳不饱和脂肪酸,是前列腺素的一种。 (4)白三烯B4(leukotriene B4,LTB4):主要在白细胞、红细胞和中性粒细胞等血细胞中合成,是有效的中性粒细胞趋化剂和促炎递质,属于白三烯类物质[27]。白三烯B4升高容易造成平滑肌收缩、支气管收缩、水肿形成、黏液分泌过多,以及炎症细胞增殖、激活和存活[28]。 (5)脂氧素 A4(lipoxin A4,LXA4):是体内重要的内源性促炎症消退介质,是花生四烯酸的代谢产物,为二十烷类化合物,属于脂氧素中的一种,脂氧素是第一类被确认具有抗炎作用的内源性脂质介质,是肺部炎症潜在的调节物质,也是炎症反应的重要“刹车信号”。脂氧素A4可减轻气道的炎症损伤、改变气道血管通透性、减轻炎性细胞渗出及促进组织修复而维护了呼吸道的结构及功能完整 [29-30]。 2.2.2 常用的氧化应激类生物标志物 见表2。"
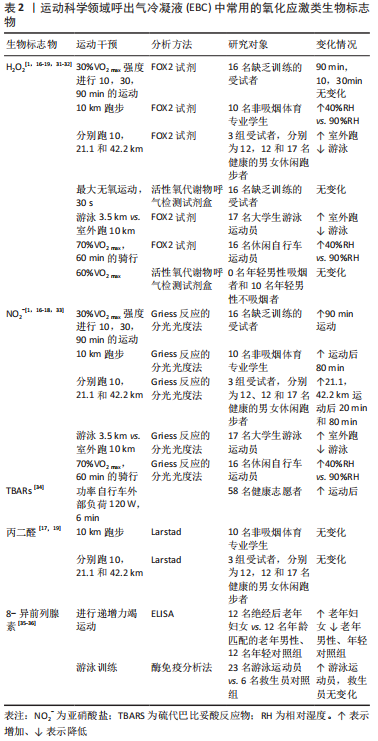

(1)过氧化氢(H2O2):被广泛接受的氧化应激的生物标志物,由氧自由基和氢离子结合产生的炎症过程和/或氧化应激的生物标志物,是超氧化物歧化酶加速的反应。在呼吸水平上,其潜在来源是肺吞噬细胞、Ⅱ型肺细胞和气道上皮细胞[37]。 (2)亚硝酸盐(NO2-):是一氧化氮(NO)的代谢物,鉴于其稳定性,可在呼出气中进行测量[37]。在运动期间,它参与支气管扩张以增加空气流量,并参与血管扩张以避免肺动脉压力增加;此外,在病理过程中,NO2-通过参与炎症现象改变氧化还原状态[38]。 (3)丙二醛:是自由基作用于脂质发生过氧化反应的氧化终产物之一,它的产生加剧膜的损伤,可以反映机体脂质过氧化速率和强度,也能间接反映组织过氧化损伤程度[39-41]。 (4)8-异前列腺素F2α(8-isoprostane F2α,8-iso-PGF2α):为二十碳烷类化合物,是前列腺素F2α的异构体。是氧自由基脂质过氧化后作用于细胞膜上的花生四烯酸的终末产物,相对稳定且特异性反映脂质过氧化水平,使其成为EBC中一种潜在且可靠地氧化应激类生物标志物[42]。 2.2.3 其他类生物标志物 见表3。"
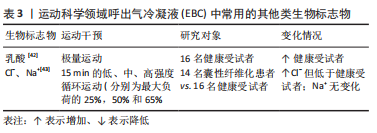

(1)乳酸:是糖无氧氧化(糖酵解)的代谢产物,是由骨骼肌、神经细胞及红细胞糖酵解产生的物质,通过肝脏代谢后经肾脏排泄,在正常情况下对于机体的酸碱度无明显影响,但剧烈的运动及体内缺氧时可导致乳酸水平升高,引起高乳酸血症甚至乳酸中毒。可反映机体细胞氧供、氧耗失衡以及组织灌注不足,是组织缺氧的标志物,常与血气分析一起评价机体酸碱代谢状态[44]。 (2)C-反应蛋白(C-reaction protein,CRP):是由肝细胞产生、能与肺炎双球菌C多糖体反应的γ球蛋白,是在机体受到感染或组织损伤时血浆中一些急剧上升的蛋白质(急性蛋白),具有很好的稳定性及精确性,是炎症和组织损伤的非特异性标志物[45]。C-反应蛋白水平的升高可能是局部炎症区域(气道)合成增强的后果,也可能由于肝细胞合成C-反应蛋白的增加造成运动后支气管收缩后炎症加剧。 2.3 EBC检测在健康人群运动中的应用 2.3.1 正常环境下运动 适度运动会升高不同人群EBC-pH值[46-47],从而减少气道的炎症水平。有研究者进一步观察了EBC中离子成分的变化,研究发现运动与氨离子浓度的升高以及pH值的同时升高相关联[47],而且运动后健康青年受试者呼出气的气体图谱发生显著变化,且呼出气中挥发性物质的特征与EBC-pH显著相关[16,43]。 研究表明在极量运动后EBC-pH值和碳酸氢盐浓度没有发生变化[48],但EBC-血栓素B2和EBC-前列腺素E2的水平显著升高[49],乳酸浓度升高的同时伴有EBC中乳酸释放速度的增加,且释放速度与耗氧量相关[50]。男性健康者以30%VO2 max强度运动后EBC-pH值[1]、积极运动者在10 km跑步后EBC-pH值没有改变[51]。竞技运动员EBC-肿瘤坏死因子α水平显著高于健康者,而白细胞介素1受体拮抗剂水平显著低于健康者,与哮喘患者相似。EBC中肿瘤坏死因子α/白细胞介素1受体拮抗剂平衡的调节异常反映出重复性剧烈运动会改变气道的炎症程度和对感染的易感性。 通过对EBC中氧化应激指标的研究发现运动会增加肺部的促氧化水平,而脂质过氧化水平没有变化[51-52]。RIEDIKER等[51]和GREENWALD等[52]研究发现进行10,21.1和42.2 km运动后、10 km运动后EBC-H2O2浓度升高,EBC-NO2-浓度具有升高趋势,10 km和42.2 km组的丙二醛浓度和pH值均无差异。ΔH2O2和ΔNO2-与比赛时间呈正相关,而ΔpH与比赛时间呈负相关;血浆?NO2-无变化,但是EBC-NO2-浓度与血浆?NO2-浓度的比值升高。适度运动对健康者EBC-H2O2和EBC-TBARs浓度均无显著影响[40],原因可能与运动负荷有关。TUESTA等[1]进一步发现了低强度运动时EBC中促氧化剂的浓度取决于运动持续时间,男性健康者以30%VO2 max强度分别运动10,30和90 min,在90 min运动后EBC-NO2-和EBC-H2O2浓度升高,总耗氧量和总通气量与NO2-和H2O2浓度呈弱相关。 组别×时间对青年进行Wingate无氧功测试的极量运动前、后EBC中的H2O2浓度具有显著的交互影响[53],而对青年以60%VO2 max中等强度运动前、后EBC-H2O2浓度并无显著交互影响[35]。 2.3.2 特定环境下运动 (1)污染因素:相关研究证明空气污染程度的变化与肺部炎症标志物的急性变化相关联,空气污染会降低EBC-pH值,污染相对轻的环境与pH值的升高相关联[54]。但青年业余跑步者在污染较轻的森林中跑步时EBC-pH值升高[15]。运动员在严重污染环境中跑步并未发生EBC-pH的酸化,较轻和较重污染环境中EBC-pH值均趋向升高,表明了运动导致了上气道炎症反应水平的降低。运动员EBC-pH值在污染环境中运动后同样没有产生急性影响,但运动前、后的pH值显著低于健康受试者,推测重复的剧烈运动可能会引起气道酸化[55]。 慢性吸入PM(空气环境中的微粒)造成安静时肺功能下降的作用机制可能与肺部的氮化/氧化应激有关。研究证明低浓度PM1运动后EBC-NO3浓度没有变化,但在高浓度PM1运动后显著降低,同样与肺功能的变化显著相关[56]。低浓度PM中运动后EBC-丙二醛增加40%,高浓度PM运动后增加了208%,造成气道脂质过氧化[57]。 在加氯消毒的泳池中游泳时吸入消毒副产物(disinfection by-products,DBPs)同样与呼吸健康受损关联,可能主要涉及氧化应激机制[58]。健康不吸烟成人在室内游泳池中游泳40 min后,尽管反映肺通透性的血清Clara细胞分泌蛋白略有升高,但肺功能和EBP-8-IsoP、细胞因子或血管内皮生长因子均无显著变化,不涉及炎症机制[59]。但竞技游泳运动员训练后EBC-8-IsoP水平显著高于基础值,且与训练后FEV1的变化率显著相关,而仅待在游泳池中没有进行运动的救生员无变化,提示在含氯泳池环境中运动导致的呼吸增强会增加气道的氧化应激水平[17]。BIKOV等[58]同样选取了大学生游泳运动员,比较了游泳和跑步对EBC中促氧化剂的影响,H2O2和NO2-的浓度在游泳后24 h低于跑步后,EBC-NO2-浓度与血浆-NO2-浓度的比值的趋势相同;在急性运动后24 h,游泳降低而跑步增加肺部的促氧化剂水平,且肺功能没有变化。 (2)低温及高湿因素:气道脱水是运动导致呼吸道产生炎症和氧化应激的主要因素之一[60]。在比较运动对游泳运动员的两种不同环境条件(室内游泳和室外跑步)的影响时,发现跑步和游泳相比,游泳降低而跑步增加肺部的促氧化剂水平,发现不同水平的环境湿度会影响促氧化剂[58]。CONTRERAS-BRICEO等[18]比较了不同相对湿度(relative humidity,RH)条件下运动对EBC指标的影响,表示高湿度阻止了高强度运动引起的呼吸过程中H2O2和NO2-的产生,并将支气管扩张效果延续运动后80 min。 运动时吸入寒冷和干燥的空气会损害运动员呼吸道上皮细胞,反复可导致气道壁结构和功能的改变。MAREK等[6]通过让男性受试者随机分别在室温(18.1±1.1) ℃或低温(-15.2±3.1) ℃环境中以75%-80%HRmax强度跑步50 min,发现与正常室温相比较,低温下运动后EBC的H2O2浓度和释放速度增加,表明气道局部炎症和氧化应激水平的增加。 (3)高原因素:常压缺氧和低压缺氧已被发现会改变抗氧化酶活性[61-62],相关研究发现男子山地自行车运动员在不同海拔各进行极量运动,EBC-丙二醛浓度在670 m处运动前、后无显著变化,而在2 160 m处运动后显著升高,运动在中等海拔增加EBC-丙二醛,支持肺氧化应激可能参与高原相关发病机制[63]。为了检验单一高原因素的影响,运动员和久坐者EBC-H2O2浓度随海拔上升而升高,返回平面后3 d仍持续在升高水平,8-IsoP水平在高原时有上升趋势,但在回到平原后立即下降[64]。 ARANEDA等[59]通过研究男性士兵在攀登高峰和返回的过程中,发现EBC-H2O2浓度从出发时670 m处到返回到2 500 m处时显著增加,丙二醛浓度从海拔670 m处到海拔3 000 m处显著上升。 高原会升高EBC中氧化应激标志物的水平,并与耐力训练无关。冬季两项运动员和久坐者分别进行高原训练和高原居住,与预期相反,两组人群期间EBC-H2O2浓度和EBC-8-iso-PGF2α水平在高原时均升高,但是在平原或高原时两组人群之间均没有差异[64]。 2.4 EBC检测在呼吸系统疾病人群运动中的应用 哮喘是一种慢性气道炎症性疾病,各种炎症细胞和递质在哮喘的发展中起作用,运动是大多数哮喘症状的重要诱因。由体育锻炼引起的气道暂时性狭窄,这一现象被称为运动性支气管狭窄 [16,43]。研究中多采用运动激发试验诱发肺功能的变化(FEV1下降> 12%-15%)来诊断运动性支气管狭窄阳性/阴性。运动性支气管狭窄是运动引起气道的暂时性收缩,其机制可能与运动中的过度换气导致气道的水分流失和/或温度改变引起气道的渗透压改变,以及气道肥大细胞或其他炎症细胞释放的递质有关。哮喘者EBC中的细胞因子和炎症递质与运动性支气管狭窄的发生及肺功能下降相关联,提示气道2 EBC检测在呼吸系统疾病人群运动中的应用。 炎症在哮喘和运动性支气管狭窄中起着重要作用[22,65]。值得注意的是,使用EBC作为评估炎症水平的工具仅针对运动性支气管狭窄阳性患者,其中的生物标志物可作为严重和急性哮喘发作时的指标[66]。 运动性支气管狭窄阳性哮喘儿童EBC-半胱氨酸白三烯浓度高于运动性支气管狭窄阴性哮喘儿童和健康儿童,且半胱氨酸白三烯浓度与运动后FEV1的下降相关(r=0.70)[20],但处于稳定期的轻度哮喘儿童在12周锻炼前、后EBC-半胱氨酸白三烯浓度都很低[67]。运动性支气管狭窄阳性哮喘患者EBC中的内皮素1水平显著高于健康者,在运动激发试验后显著升高,运动后24 h恢复到初始水平,但运动对运动性支气管狭窄阴性哮喘患者和健康者无显著影响[68]。哮喘患者EBC-肿瘤坏死因子α的基础水平与运动激发试验后FEV1的下降显著相关(r=0.64)。运动性支气管狭窄阳性哮喘儿童EBC中细胞因子的浓度同样取决于运动后FEV1的下降情况,其中的单核细胞趋化蛋白1和白细胞介素16水平显著高于运动性支气管狭窄阴性哮喘儿童[69]。 运动性支气管狭窄阳性哮喘儿童运动后即刻EBC-脂氧素A4水平显著升高,且脂氧素A4水平与运动后FEV1的下降之间呈显著负相关(r=-0.50),推测脂氧素A4水平升高是为了抵抗支气管狭窄和抑制急性炎症反应,并导致了运动性支气管狭窄后发生的支气管扩张[34]。 气道酸碱度的急性变化在运动性支气管狭窄的发生中起作用。运动性支气管狭窄阳性哮喘者运动期间EBC-pH值显著下降,而无运动性支气管狭窄哮喘者和健康者无变化。运动性支气管狭窄阳性哮喘患者EBC-pH值的下降与支气管痉挛程度相关(r=0.68),建议将EBC-pH值作为气道酸碱度的替代指标[16]。但处于稳定期的轻度哮喘儿童在12周运动锻炼后EBC-pH值下降[68],与在高中生运动员中观察到的现象一致[55]。 运动对囊性纤维化患者的系统性益处与改善受损的离子调节水平有关,EBC有可能成为评估肺部离子调节水平的可行方法并应用于临床。囊性纤维化患者EBC-Cl-的基础浓度低于健康者,中等强度(50%的最大负荷)运动使得囊性纤维化患者EBC-Cl-的浓度增加了4倍,与注射沙丁胺醇后的变化相似,提示运动可以改善囊性纤维化患者的离子调节水平[70]。"

| [1] TUESTA M, ALVEAR M, CARBONELL T, et al. Effect of exercise duration on pro-oxidants and pH in exhaled breath condensate in humans. J Physiol Biochem. 2016;72(2):353-360. [2] DING S, ZHONG C. Exercise and Asthma. Adv Exp Med Biol. 2020;1228: 369-380. [3] ANDERSON SD, KIPPELEN P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol. 2008;122(2):225-235. [4] CARLSEN KH. Sports in extreme conditions: The impact of exercise in cold temperatures on asthma and bronchial hyper-responsiveness in athletes. Br J Sports Med. 2012;46(11):796-799. [5] NATIONAL INSTITUTE OF HEALTH N H, LUNG, AND BLOOD INSTITUTE. Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA). http://www.ginasthmaorg, 2006. [6] MAREK E, VOLKE J, MÜCKENHOFF K, et al. Exercise in cold air and hydrogen peroxide release in exhaled breath condensate. Adv Exp Med Biol. 2013;756:169-177. [7] KIPPELEN P, FITCH K D, ANDERSON SD, et al. Respiratory health of elite athletes- preventing airway injury: a critical review. Br J Sports Med. 2012;46(7):471-476. [8] KUROWSKI M, JURCZYK J, OLSZEWSKA-ZIABER A, et al. A similar pro/anti-inflammatory cytokine balance is present in the airways of competitive athletes and non-exercising asthmatics. Adv Med Sci. 2018;63(1):79-86. [9] PATSIRIS S, EXARCHOS T, VLAMOS P. Exhaled Breath Condensate(EBC): Is It a Viable Source of Biomarkers for Lung Diseases?. Adv Exp Med Biol. 2020;1195:13-18. [10] MANISCALCO M, FUSCHILLO S, PARIS D, et al. Clinical metabolomics of exhaled breath condensate in chronic respiratory diseases. Adv Clin Chem. 2019;88:121-149. [11] 徐百川,李艺婷,赵虎雷,等.呼出气冷凝液生物标志物检测在呼吸系统疾病中的应用现状及前景[J].中国全科医学,2022,25(2): 139-144. [12] SIDORENKO GI, ZBOROVSKI EI, LEVINA DI. Surface-active properties of the exhaled air condensate (a new method of studying lung function). Ter Arkh. 1980;52(3):65-68. [13] 孙健,沈巨信.简易呼出气冷凝液收集装置的设计及临床应用[J].实用医学杂志,2010,26(17):3233-3235. [14] 陈宏,黄莺,余荣环,等.呼出气冷凝液收集器的设计及其临床应用[J].中国临床医学,2013,20(4):498-499. [15] MATHEUS C, NAKAGAWA NK, DIVA S, et al. Aerobic exercise in polluted urban environments:effects on airway defense mechanisms in young healthy amateur runners. J Breath Res. 2016;10(4):046018. [16] ARANEDA OF, FELIPE CB, GABRIEL C, et al. Swimming versus running: effects on exhaled breath condensate pro-oxidants and pH. Eur J Appl Physiol. 2018;118(11):2319-2329. [17] ARANEDA OF, GUEVARA AJ, CONTRERAS C, et al. Exhaled breath condensate analysis after long distance races. Int J Sports Med. 2012; 33(12):955-961. [18] CONTRERAS-BRICEO F, ESPINOSA-RAMIREZ M, VISCOR G, et al. Humidity prevents the exercise-induced formation of hydrogen peroxide and nitrite in exhaled breath condensate in recreational cyclists. Eur J Appl Physiol. 2020;120(10):2339-2348. [19] ARANEDA OF, URBINA-STAGNO R, TUESTA M, et al. Increase of pro-oxidants with no evidence of lipid peroxidation in exhaled breath condensate after a 10-km race in non-athletes. J Physiol Biochem. 2014;70(1):107-115. [20] CARRARO S, CORRADI M, ZANCONATO S, et al. Exhaled breath condensate cysteinyl leukotrienes are increased in children with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005; 115(4):764-770. [21] Morissette MC, Murray N, Turmel J, et al. Increased exhaled breath condensate 8-isoprostane after a swimming session in competitive swimmers. Eur J Sport Sci. 2016;16(5):569-576. [22] STELMACH I, ZACZENIUK M, SZTAFIŃSKA A, et al. Serum tryptase level and inflammatory markers in exhaled breath condensate of children with exercise-induced symptoms. Allergy Asthma Proc. 2016;37(5):84-92. [23] TAHAN F, EKE GH, BICICI E, et al. Increased postexercise lipoxin A4 levels in exhaled breath condensate in asthmatic children with exercise-induced bronchoconstriction. J Investig Allergol Clin Immunol. 2016;26(1):19-24. [24] CARLSEN KH. Asthma, airway inflammation and epithelial damage in elite athletes. Eur Respir J. 2009;33(4):713-714. [25] SOMA T, UCHIDA Y, NAKAGOME K, et al. Eicosanoids seasonally impact pulmonary function in asthmatic patients with Japanese cedar pollinosis. Allergol Int. 2020;69(4):594-600. [26] UCHIDA Y, SOMA T, NAKAGOME K, et al. Implications of prostaglandin D2 and leukotrienes in exhaled breath condensates of asthma. Ann Allergy Asthma Immunol. 2019;123(1):81-88.e1. [27] KUMAR TC, REDDY KV, ANURADHA D, et al. Enhanced production of LTB4 and free radicals in rat lung by exhaustive physical exercise. Biochem Mol Biol Int. 2010;41(3):641-646. [28] ALBERCA-CUSTODIO RW, GREIFFO FR, MACKENZIE B, et al. Aerobic Exercise Reduces Asthma Phenotype by Modulation of the Leukotriene Pathway. Front Lmmunol. 2016;7:237. [29] SERHAN CN. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis:an update and role in anti-inflammation and pro-resolution. Prostaglandins Other Lipid Mediat. 2002;68:433-455. [30] NAN C, ARITA M, SERHAN CN. Anti-inflammatory circuitry: lipoxin,aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3-4):163-177. [31] TAITO S, SEKIKAWA K, DOMEN S, et al. Pulmonary oxidative stress is induced by maximal exercise in young cigarette smokers. Nicotine Tob Res. 2012;14(2):243-247. [32] TAITO S, DOMEN S, SEKIKAWA K, et al. Cigarette Smoking does not Induce Plasma or Pulmonary Oxidative Stress after Moderate-intensity Exercise. J Phys Ther Sci. 2014;26(3):413-425. [33] MAREK E, PLATEN P, VOLKE J, et al. Hydrogen peroxide release and acid-base status in exhaled breath condensate at rest and after maximal exercise in young, healthy subjects. Eur J Med Res. 2009;14(14):134-139. [34] NOWAK D, KALUCKA S, BIAASIEWICZ P, et al. Exhalation of H2O2 and thiobarbituric acid reactive substances (TBARs) by healthy subjects. Free Radic Biol Med. 2001;30(2):178-186. [35] MORISSETTE MC, MURRAY N, TURMEL J, et al. Increased exhaled breath condensate 8-isoprostane after a swimming session in competitive swimmers. Eur J Sport Sci. 2016;16(5):569-576. [36] KURTI SP, EMERSON SR, SMITH JR, et al. Older women exhibit greater airway 8-isoprostane responses to strenuous exercise compared with older men and younger controls. Appl Physiol Nutr Metab. 2018;43(5): 497-503. [37] LIANG Y, YELIGAR SM, BROWN L. Exhaled Breath Condensate: A Promising Source for Biomarkers of Lung Disease. ScientificWorldJournal. 2012;2012:217518. [38] RICCIARDOLO FL, STERK PJ, GASTON B, et al. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84(3):731-765 [39] DEL RIO D, STEWART AJ, PELLEGRINI N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15(4):316-328. [40] FRANKEL EN, NEFF WE. Formation of malonaldehyde from lipid oxidation products. Biochim Biophys Acta. 1983;754:227. [41] NIEDERNHOFER LJ, DANIELS JS, ROUZER CA, et al. Malondialdehyde,a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278(33):31426. [42] NAKANISHI K, TAJIMA F, NAKAMURA A, et al. Effects of hypobaric hypoxia on antioxidant enzymes in rats. J Physiol. 1995;489(3):869-876. [43] HENDERSON EM, GASTON B. SNOR and wheeze: the asthma enzyme? Trends Mol Med. 2005;11(11):481-484. [44] HIRA HS, SHUKLA A, KAUR A, et al. Serum uric acid and lactate levels among patients with obstructive sleep apnea syndrome:which is a better marker of hypoxemia? Ann Saudi Med. 2012;32(1):37-42. [45] GHELLI F, PANIZZOLO M, GARZARO G, et al. Inflammatory Biomarkers in Exhaled Breath Condensate: A Systematic Review. Int J Mol Sci. 2022; 23(17):9820. [46] HUNT JF, ERWIN E, PALMER L, et al. Expression and activity of pH-regulatory glutaminase in the human airway epithelium. Am J Respir Crit Care Med. 2002;165(1):101-107. [47] ZHANG J, TONG Z, KIPEN H, et al. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Res Rep Health Eff Inst. 2013;(174):165-174. [48] SHOMAN Y, WILD P, HEMMENDINGER M, et al. Reference Ranges of 8-Isoprostane Concentrations in Exhaled Breath Condensate (EBC): A Systematic Review and Meta-Analysis. Int J Mol Sci. 2020;21(11):3822. [49] FONT-RIBERA L, KOGEVINAS M, ZOCK JP, et al. Short-Term Changes in Respiratory Biomarkers after Swimming in a Chlorinated Pool. Environ Health Pesp. 2010;118(11):1538-1544. [50] RUNDELL KW, SLEE JB, CAVISTON R, et al. Decreased Lung Function After Inhalation of Ultrafine and Fine Particulate Matter During Exercise is Related to Decreased Total Nitrate in Exhaled Breath Condensate. Inhal Toxicol. 2008;20(1):1-9. [51] RIEDIKER M, DANUSER B. Exhaled breath condensate pH is increased after moderate exercise. J Aerosol Med. 2007;20(1):13. [52] GREENWALD R, FERDINANDS JM, TEAGUE WG. Ionic determinants of exhaled breath condensate pH before and after exercise in adolescent athletes. Pediatr Pulmonol. 2010;44(8):768-777. [53] BIKOV A, LAZAR Z, SCHANDL K, et al. Exercise changes volatiles in exhaled breath assessed by an electronic nose. Acta Physiol Hung. 2011;98(3):321-328. [54] MERKER MP, PITT BR, CHOI AM, et al. Lung redox homeostasis:emerging concepts. Am J Physiol-Lung C. 2000;279(3):413-417. [55] FERDINANDS JM, CRAWFORD C, GREENWALD R, et al. Breath acidification in adolescent runners exposed to atmospheric pollution: A prospective, repeated measures observational study. Environ Health. 2008;7:10. [56] HOSHIKAWA Y, ONO S, SUZUKI S, et al. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol. 2001;90(4):1299-1306. [57] ARANEDA OF, CARBONELL T, TUESTA M. Update on the mechanisms of pulmonary inflammation and oxidative imbalance induced by exercise. Oxid Med Cell Longev. 2016;2016:4868536. [58] BIKOV A, GALFFY G, TAMASI L, et al. Exhaled breath condensate pH decreases during exercise-induced bronchoconstriction. Respirology. 2014;19(4):563-569. [59] ARANEDA OF, GARCIA C, LAGOS N, et al. Lung oxidative stress as related to exercise and altitude. Lipid peroxidation evidence in exhaled breath condensate:a possible predictor of acute mountain sickness. Eur J Appl Physiol. 2005;95(5-6):383-390. [60] HEINICKE I, BOEHLER A, RECHSTEINER T, et al. Moderate altitude but not additional endurance training increases markers of oxidative stress in exhaled breath condensate. Eur J Appl Physiol. 2009;106(4):599. [61] 张海婧,徐春雨,胡小键,等.呼出气冷凝液中尿素的高效液相色谱-串联质谱测定法[J].环境与健康杂志,2017,34(2):161-163. [62] BONSIGNORE MR, GRUTTA SL, CIBELLA F, et al. Effects of exercise training and montelukast in children with mild asthma]. Med Sci Sport Exer. 2008;40(3):405. [63] ZIETKOWSKI Z, SKIEPKO R, TOMASIAK-LOZOWSKA MM, et al. Endothelin-1 in exhaled breath condensate of allergic asthma patients with exercise-induced bronchoconstriction. Resp Res. 2007;82(2):169 [64] MAJAK P, JERZYŃSKA J, BOJO M, et al. Cytokine profiling in exhaled breath condensate after exercise challenge in asthmatic children with post-exercise symptoms. Arch Med Sci. 2016;12:778-784. [65] WHEATLEY CM, BAKER SE, MORGAN MA, et al. Moderate intensity exercise mediates comparable increases in exhaled chloride as albuterol in individuals with cystic fibrosis. Respir Med. 2015;109(8):1001-1011. [66] TAITO S, SEKIKAWA K, DOMEN S, et al. Pulmonary oxidative stress is induced by maximal exercise in young cigarette smokers. Nicotine Tob Res. 2012;14(2):243-247. [67] 吕晓红,陈星海,李洋,等.呼出气冷凝液收集影响因素的探讨[J].中国实验诊断学,2011,15(8):1346-1348. [68] 吕晓红,李丹,袁海波,等.影响呼出气冷凝液pH值、一氧化氮、过氧化氢测定因素的探讨[J].中国实验诊断学,2012,16(10):1833-1835. [69] MAREK E, MüCKENHOFF K, STRECKERT HJ, et al. Measurements of L-lactate and H2O2 in exhaled breath condensate at rest and mild to moderate exercise in young and healthy subjects. Pneumologie. 2008;62(9):541. [70] CHEN X, CHEN W, WANG Y, et al. Responses of healthy young males to fine-particle exposure are modified by exercise habits: a panel study. Environ Health. 2018;17(1):88. [71] HORVATH I, BARNES PJ, LOUKIDES S, et al. A European Respiratory Society technical standard:exhaled biomarkers in lung disease. Eur Respir J. 2017;26(3):523-548. [72] RUNDELL KW, SLEE JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122(2):238-246. [73] 熊小明,童国强,付云杰,等.支气管哮喘-慢性阻塞性肺疾病重叠综合征患者呼出气冷凝液和血清8-isoPG、MPO、LTB4、IL-6的测定及临床意义[J].实用医学杂志,2018,34(4):649-652. [74] PUCSOK JM, GYOERE I, ARGAY K, et al. Effect of exercise on levels of cyclo-oxygenase mediators in exhaled breath condensate in elite athletes. J Sports Med Phys Fitness. 2007;47(2):223-227. [75] LINDSAY A, COSTELLO JT. Realising the potential of urine and saliva as diagnostic tools in sport and exercise medicine. Sports Med. 2016; 47(1):11-31. [76] CAVALCANTE DE SÁ M, NAKAGAWA NK, SALDIVA DE ANDRÉ CD, et al. Aerobic exercise in polluted urban environments: effects on airway defense mechanisms in young healthy amateur runners. J Breath Res. 2016;10(4):046018. [77] HORVATH I, BARNES PJ, LOUKIDES S, et al. A European Respiratory Society technical standard:exhaled biomarkers in lung disease. Eur Respir J. 2017;49(4):1600965. [78] CARPAGNANO GE, LACEDONIA D, FOSCHINO-BARBARO MP. Non-invasive study of airways inflammation in sleep apnea patients. Sleep Med Rev. 2011;15(5):317-326. [79] MUTLU G, GAREY K, ROBBINS R, et al. Collection and analysis of exhaled breath condensate in humans. Am J Respir Crit Care Med. 2001;164(5):731-737. |
| [1] | Yang Hongxia, Shi Qinghong, Weng Minhua, Zhang Jianyong, Zhao Jianjun, Chen Ling. Effect of human amniotic mesenchymal stem cell transplantation on airway goblet cell proliferation and mucin 5ac expression in asthmatic mice [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(25): 4050-4055. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 433
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 353
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||