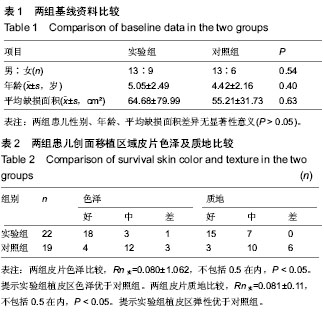
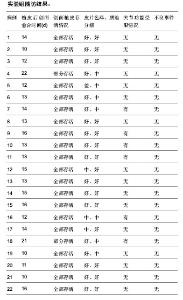
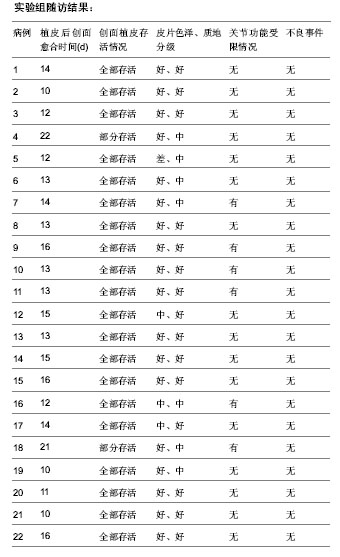
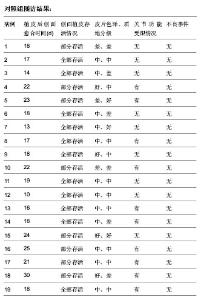
| [1] 王康建,曾睿,但卫华,等.基于天然高分子材料的组织工程化皮肤支架材料[J].生物医学工程与临床,2009,13(2):161-166.
[2] Eisenbud D, Hettrick H, Evans C, et al. The interdisciplinary CWS,5 years later.Adv Skin Wound Care. 2004;17(7):336-340.
[3] 孙永华.积极开展创面覆盖物研究,促进伤口高质量修复[J].中华损伤与修复杂志:电子版,2011,6(1):6-11.
[4] Dantzer E, Queruel P, Salinier L, et al. Dermal regeneration template for deep hand burns:clinical utility for both early grafting and reconstructive surgery. Br J Plast Surg. 2003; 56(8): 764-774.
[5] Clayman MA, Clayman SM, Mozingo DW. The use of collagen-glycosaminoglycan copolymer (Integra) for the repair of hypertrophic scars and keloids . Burn Care Res. 2006; 27(3):404-409.
[6] 朱希山,曾丽芬,赵春华.组织工程化人工皮肤研究的新进展[J].中国组织工程研究与临床康复,2007,11(6):1145-1148.
[7] Soekjma K,Nozaki M,Sasaki K,et al. Reconstruction of bum de-formity using artificial dermis combined with split-skin grafting. Burns. 1997;23(6):501-504.
[8] 姜笃银,陈壁,徐明达,等.冷冻异体胎儿真皮-自体表皮复合移植在Ⅲ度烧伤切痂创面的应用[J]. 第四军医大学学报,1997,18(5): 461-463.
[9] 陈壁,贾赤宁,徐明达,等.自体皮源奇缺条件下瘢痕孪缩畸形的晚期临床修复[J]. 中华烧伤杂志,2003,9(6):361-364.
[10] 肖军,邱林,傅跃先,等.封闭式负压引流技术在儿童感染创面修复中的应用[J].重庆医科大学学报,2011,36(7):875-877.
[11] 姚元章,黄显凯,麻晓林,等. 负压封闭技术治疗创伤后软组织缺损创伤[J].创伤外科杂志,2002,49(1):9-12.
[12] 陈彬,梁国荣,首家保,等.人工真皮复合自体刃厚皮片修复全层皮肤缺损创面的临床研究[J].中国美容医学,2012,21(2):208-210.
[13] Wild T, Stremitzer S, Budzanowski A, et al. Defi nition of effi ciency in vacuum therapy-a randomised controlled trial comparing with V.A.C.Therapy. Int Wound.2008;5(5): 641-647.
[14] 张陈威,柳大烈,梁智,等.封闭式负压引流与人工真皮联合应用治疗下肢慢性溃疡[J].中国修复重建外科杂志,2013,27(8): 1023-1024.
[15] 汪璟,丘宏伟,李远景,等.负压封闭引流技术在骨科创面修复中的临床应用[J].实用临床医学,2013,14(3):31-32.
[16] 梁尊鸿,潘云川,徐家钦,等. 异体异种脱细胞真皮与自体刃厚皮复合移植的比较[J]. 中国组织工程研究与临床康复,2009,
13(41):8048-8052.
[17] 孙维国,樊星.脱细胞异体真皮修复烧伤瘢痕的临床观察[J].中国美容医学,2008,17(7):974-975.
[18] Figus A,Leon-Villapalos J,Philp B,et al.Severe multiple extensive postburn contractures: a simultaneous approach with total scar tissue excision and resurfacing with dermal regeneration template. J Burn Care Res. 2007;28(6):913-917.
[19] Silvestro C , Ferdinando C, Angela DC, et al. The use of a dermal substitute and thin skin grafts in the cure of "Complex" leg ulcers.Dermatol Surg.2009;35:195-200.
[20] 陈晓东,冯祥生,阮树斌,等. 脱细胞(猪)真皮支架在深度烧伤创面及瘢痕整形中的临床应用[J].解放军医学杂志,2010,35(8): 966-968.
[21] Yannas IV, Burke JF, Gordon PL, et al. Design of an artificial skin.II. Control of chemical composition. Biomed Mater Res. 1980;14(2):107-132.
[22] 方洪松,王虎,甘经岳,等.人工真皮复合自体薄皮移植治疗皮肤软组织缺损[J].中国组织工程研究与临床康复,2011,15(24): 4503-4506.
[23] 田彭,周业平,张国安.人工真皮修复软组织缺损20例[J].中国组织工程研究与临床康复,2009,13(53):10573-10576.
[24] 余继超,邱加崇,彭文要,等.人工真皮修复慢性创面疗效观察[J].国际医药卫生导报,2012,18(15):2198-2200.
[25] 陈柏秋,彭文要,邱加崇,等.人工真皮在皮肤撕脱伤治疗中的临床应用[J].中华损伤与修复杂志:电子版,2013,8(5):515-517.
[26] 向小燕,周国富,王珺,等.皮能快愈敷料在整形手术创面修复中的应用[J].华西医学,2013, 28(6):818-820.
[27] 王晓红,李叶扬,黄峻,等.植入式人工真皮联合自体刃厚皮片治疗烧伤瘢痕的效果[J].广东医学,2013,34(13):2035-2036.
[28] Takemoto S,Morimoto N,Kimura Y,et al. Preparation of collagen/gelatin aponge scaffold for sustained release of bFGF. Tissue Eng Part A. 2008;14(10):1629-1638.
[29] 陈欣,副岛一孝,野琦斡弘,等. 成纤维细胞移植促进人工真皮内血管新生的研究[J]. 中国修复重建外科杂志, 2004,18(3): 205-208.
[30] Ehrenreich M, Ruszczak Z. Update on dermal substitutes. Acta Dermatovenerol Croat. 2006;14(3):172-187.
[31] 张志民,夏成德.脱细胞异体真皮在瘢痕畸形中的应用[J].中国实用医刊,2009,36(15):30-31.
[32] 倪俊,顾海峰,许献荣,等.47例烧伤外科儿童手术的临床分析[J].局解手术学杂志,2013,22(6):635-639. |