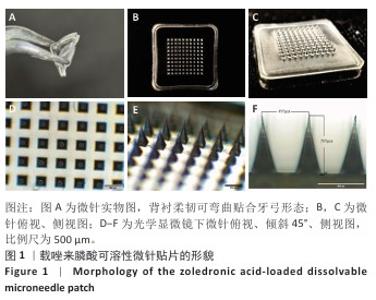
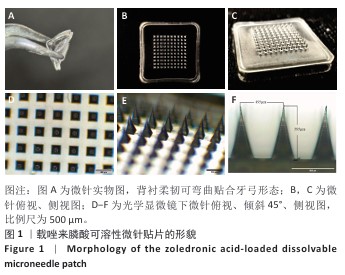
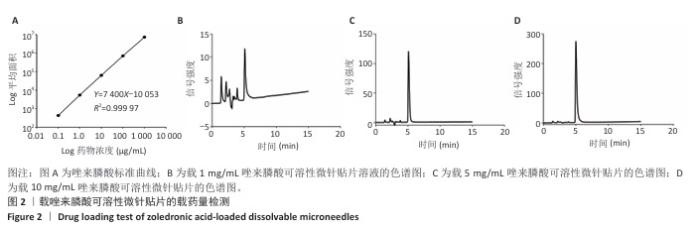
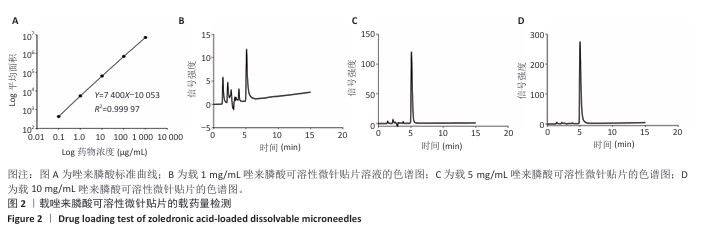
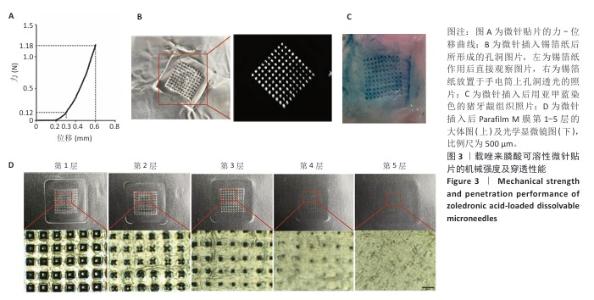
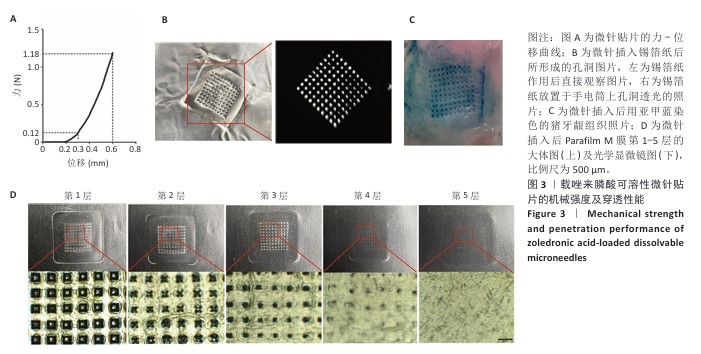
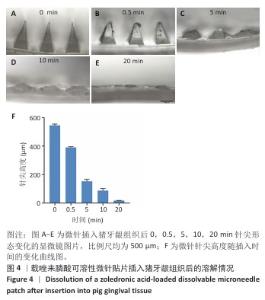
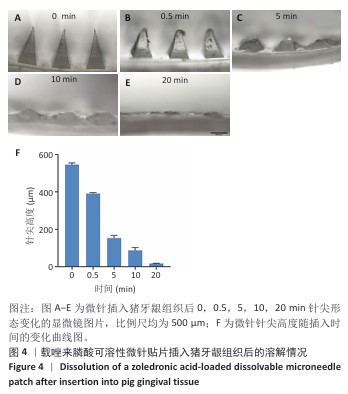
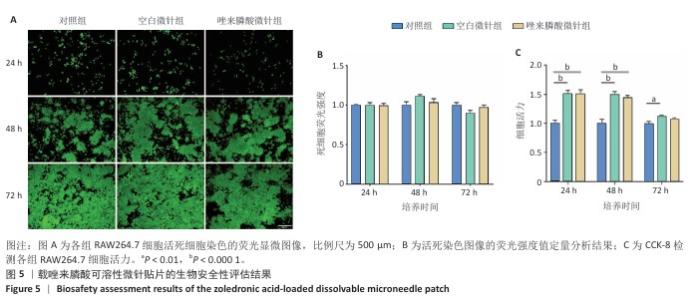
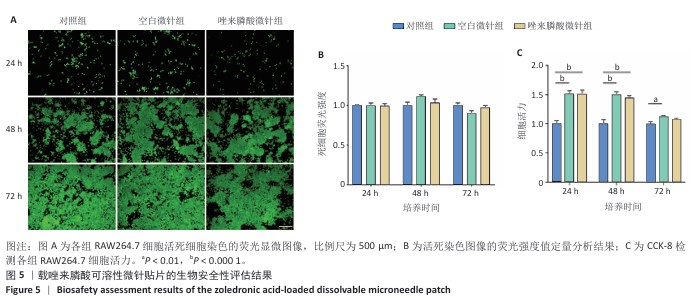
[1] SCHWARZ F, DERKS J, MONJE A, et al. Peri-implantitis. J. Clin. Periodontol. 2018;45(S20):S246-S266.
[2] CHEN L, TAO C, ZHANG D, et al. Osteogenic Microenvironment Restoration around Dental Implants Induced by an Injectable MXene-Based Hydrogel. ACS Appl. Nano Mater. 2024;7(11): 13071-13088.
[3] HU B, QIAO W, CAO Y, et al. A sono-responsive antibacterial nanosystem co-loaded with metformin and bone morphogenetic protein-2 for mitigation of inflammation and bone loss in experimental peri-implantitis. Front Bioeng Biotechnol. 2024;12:1410230.
[4] ABE K, YOSHIMURA Y, DEYAMA Y, et al. Effects of bisphosphonates on osteoclastogenesis in RAW264.7 cells. Int J Mol Med. 2012;29(6): 1007-1015.
[5] NANCOLLAS GH, TANG R, PHIPPS RJ, et al. Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone. 2006;38(5):617-627.
[6] DIKICIER E, KARAÇAYLı Ü, DIKICIER S, et al. Effect of systemic administered zoledronic acid on osseointegration of a titanium implant in ovariectomized rats. J Craniomaxillofac Surg. 2014;42(7):1106-1111.
[7] 刘官娟,宋娜,霍花,等.唑来膦酸调控NLRP3信号通路抑制脂多糖诱导的破骨细胞分化[J].中国组织工程研究,2023,27(29): 4677-4683.
[8] 宋娜,刘官娟,程余婷,等.唑来膦酸促进去势大鼠牙槽骨的成骨作用[J].中国组织工程研究,2023,27(28): 4494-4501.
[9] GRADOS D, MARTÍNEZ-MORILLO M, ERRA A, et al. THU0395 Zoledronic Acid: Patterns of Prescription and Side Effects in 204 Patients. Ann Rheum Dis. 2013;72(Suppl 3):A299-A299.
[10] ZHANG Y, WU M, TAN D, et al. A dissolving and glucose-responsive insulin-releasing microneedle patch for type 1 diabetes therapy. J Mater Chem B. 2021;9(3):648-657.
[11] AUNG NN, NGAWHIRUNPAT T, ROJANARATA T, et al. HPMC/PVP Dissolving Microneedles: a Promising Delivery Platform to Promote Trans-Epidermal Delivery of Alpha-Arbutin for Skin Lightening. AAPS PharmSciTech. 2019;21(1):25.
[12] SHI H, HUAI S, WEI H, et al. Dissolvable hybrid microneedle patch for efficient delivery of curcumin to reduce intraocular inflammation. Int J Pharm. 2023;643:123205.
[13] SHI J, MA Q, SU W, et al. Effervescent cannabidiol solid dispersion-doped dissolving microneedles for boosted melanoma therapy via the “TRPV1-NFATc1-ATF3” pathway and tumor microenvironment engineering. Biomater Res. 2023;27(1):48.
[14] YERNENI SS, YALCINTAS EP, SMITH JD, et al. Skin-targeted delivery of extracellular vesicle-encapsulated curcumin using dissolvable microneedle arrays. Acta Biomater. 2022;149:198-212.
[15] GUO X, ZHU T, YU X, et al. Betamethasone-loaded dissolvable microneedle patch for oral ulcer treatment. Colloids Surf. B Biointerfaces. 2023;222:113100.
[16] MANIMARAN R, PATEL KD, LOBO VM, et al. Buccal mucosal application of dissolvable microneedle patch containing photosensitizer provides effective localized delivery and phototherapy against oral carcinoma. Int J Pharm. 2023;640:122991.
[17] CHUN YY, TAN WWR, VOS MIG, et al. Scar prevention through topical delivery of gelatin-tyramine-siSPARC nanoplex loaded in dissolvable hyaluronic acid microneedle patch across skin barrier. Biomater Sci. 2022;10(14):3963-3971.
[18] FRASHERI I, TSAKIRIDOU ND, HICKEL R, et al. The molecular weight of hyaluronic acid influences metabolic activity and osteogenic differentiation of periodontal ligament cells. Clin Oral Investig. 2023; 27:5905-5911.
[19] MARRA M, SALZANO G, LEONETTI C, et al. New self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: a comparative study. Biotechnol Adv. 2011;30(1):302-309.
[20] AL-BADRY AS, AL-MAYAHY MH, SCURR DJ. Enhanced Transdermal Delivery of Acyclovir via Hydrogel Microneedle Arrays. J Pharm Sci. 2023;112(4):1011-1019.
[21] ZAREI CHAMGORDANI N, ASIAEI S, GHORBANI-BIDKORPEH F, et al. A Long-Lasting Triamcinolone-Loaded Microneedle Patch for Prolonged Dermal Delivery. Iran J Pharm Res. 2024;23(1):e138857.
[22] GHAZI RF, AL-MAYAHY MH. Levothyroxine sodium loaded dissolving microneedle arrays for transdermal delivery. ADMET DMPK. 2022; 10(3):213-230.
[23] SHEN L, HU J, YUAN Y, et al. Photothermal-promoted multi-functional gallic acid grafted chitosan hydrogel containing tannic acid miniaturized particles for peri-implantitis. Int J Biol Macromol. 2023;253(Pt 6):127366.
[24] ZHOU Z, ZHANG Q, WANG Y. Preparation and characterization of antibacterial and anti-inflammatory hyaluronic acid-chitosan-dexamethasone hydrogels for peri-implantitis repair. J Biomater Appl. 2021;36(7):1141-1150.
[25] 车宁.唑来膦酸的作用机制及临床应用[J].首都食品与医药,2016, 23(18):75-76.
[26] 霍花,刘官娟,宋娜,等.唑来膦酸干预去势大鼠牙槽骨骨代谢及核苷酸结合寡聚化结构域样受体蛋白3炎症小体表达的变化[J].中国组织工程研究,2022,26(17):2660-2666.
[27] REEM KHALED W, BAHER AD, MOHAMMED M. FRESH 3D printing of zoledronic acid-loaded chitosan/alginate/hydroxyapatite composite thermosensitive hydrogel for promoting bone regeneration. Int J Pharm. 2024;667(Pt A):124898.
[28] SIVERINO C, TIRKKONEN-RAJASALO L, FREITAG L, et al. Restoring implant fixation strength in osteoporotic bone with a hydrogel locally delivering zoledronic acid and bone morphogenetic protein 2. A longitudinal in vivo microCT study in rats. Bone. 2024;180:117011.
[29] ALKIN O, BUSRA K, MOHAMMADREZA G, et al. Injectable carboxymethyl chitosan/oxidized dextran hydrogels containing zoledronic acid modified strontium hydroxyapatite nanoparticles. RSC Adv. 2025;15(6): 4014-4028.
[30] MAKVANDI P, KIRKBY M, HUTTON ARJ, et al. Engineering Microneedle Patches for Improved Penetration: Analysis, Skin Models and Factors Affecting Needle Insertion. Nanomicro Lett. 2021;13(1):93.
[31] LOIZIDOU EZ, INOUE NT, ASHTON-BARNETT J, et al. Evaluation of geometrical effects of microneedles on skin penetration by CT scan and finite element analysis. Eur J Pharm Biopharm. 2016;107:1-6.
[32] WATCHORN J, CLASKY AJ, PRAKASH G, et al. Untangling Mucosal Drug Delivery: Engineering, Designing, and Testing Nanoparticles to Overcome the Mucus Barrier. ACS Biomater Sci Eng. 2022;8(4): 1396-1426.
[33] KOU Y, LI C, YANG P, et al. The W9 peptide inhibits osteoclastogenesis and osteoclast activity by downregulating osteoclast autophagy and promoting osteoclast apoptosis. J Mol Histol. 2022;53(1):27-38.
[34] CAI L, QIN X, XU Z, et al. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega. 2019;4(7):12036-12042. |