Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (32): 6927-6938.doi: 10.12307/2025.936
Previous Articles Next Articles
Exploration of biomarkers for moyamoya disease and analysis of traditional Chinese medicine targets#br#
#br#
Zhou Rulin1, Hu Yuanzheng2, Wang Zongqing2, Zhou Guoping3, Zhang Baochao4, Xu Qian2, Bai Fanghui5
- 1Graduate School of Xinxiang Medical University, Xinxiang 453000, Henan Province, China; 2School of Life Science, Nanyang Normal University, Nanyang 473000, Henan Province, China; 3Department of Neurosurgery, 4Department of Neurology, 5Department of Medical Service, Nanyang Central Hospital, Nanyang 473000, Henan Province, China
-
Received:2024-09-27Accepted:2024-12-06Online:2025-11-18Published:2025-04-27 -
Contact:Zhang Baochao, Professor, Master’s supervisor, Department of Neurology, Nanyang Central Hospital, Nanyang 473000, Henan Province, China Co-corresponding author: Xu Qian, Associate professor, Master’s supervisor, School of Life Science, Nanyang Normal University, Nanyang 473000, Henan Province, China Co-corresponding author: Bai Fanghui, Associate professor, Master’s supervisor, Department of Medical Service, Nanyang Central Hospital, Nanyang 473000, Henan Province, China -
About author:Zhou Rulin, Master, Physician, Nanyang Central Hospital of Xinxiang Medical University, Xinxiang 453000, Henan Province, China -
Supported by:Henan Provincial Key Research and Development Special Project, No. 231111310900 (to BFH); Henan Provincial Science and Technology Tackling Project, No. 232102310198 (to BFH); Nanyang Basic and Foreword Technology Research Special Project, No. 23JCQY1007 (to BFH)
CLC Number:
Cite this article
Zhou Rulin, Hu Yuanzheng, Wang Zongqing, Zhou Guoping, Zhang Baochao, Xu Qian, Bai Fanghui. Exploration of biomarkers for moyamoya disease and analysis of traditional Chinese medicine targets#br#
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

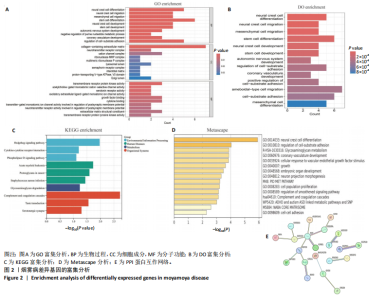
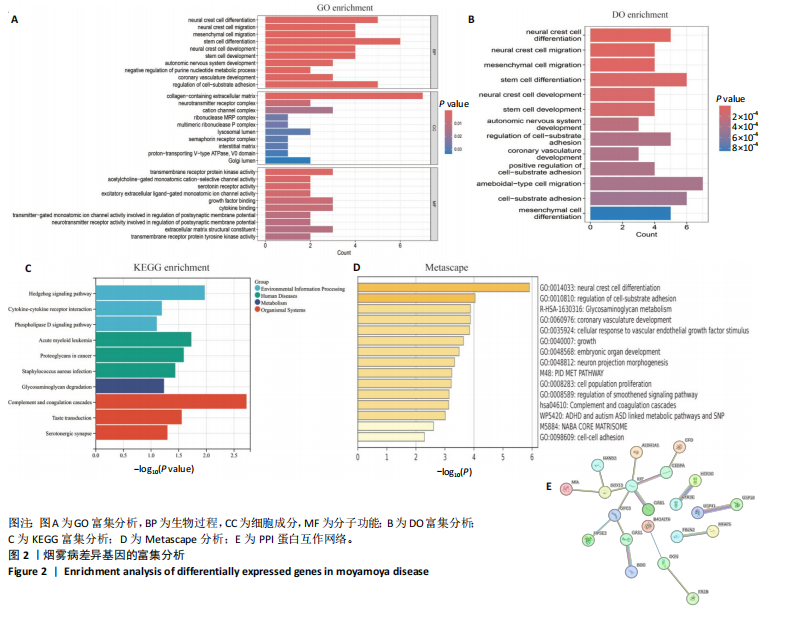
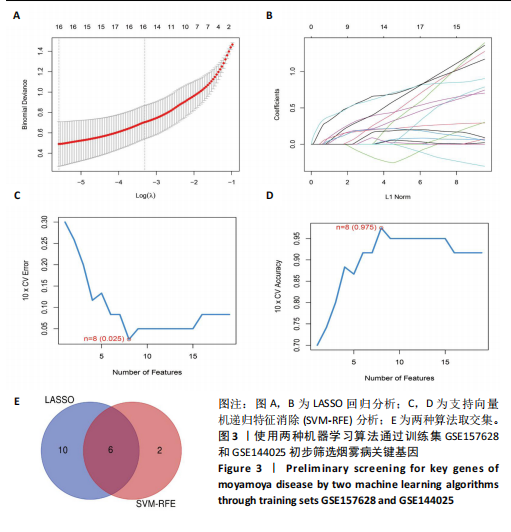
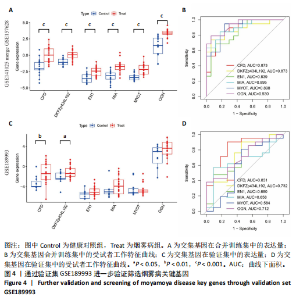
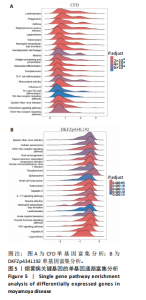
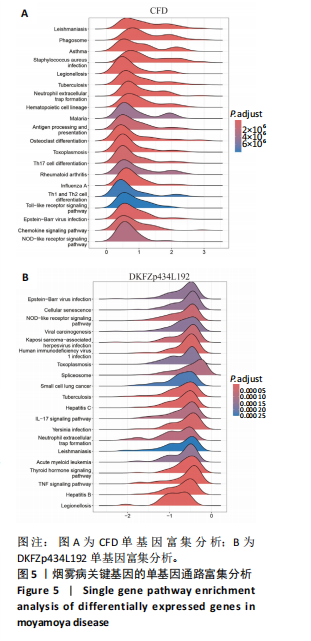
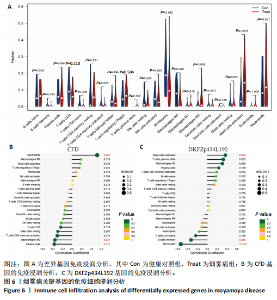
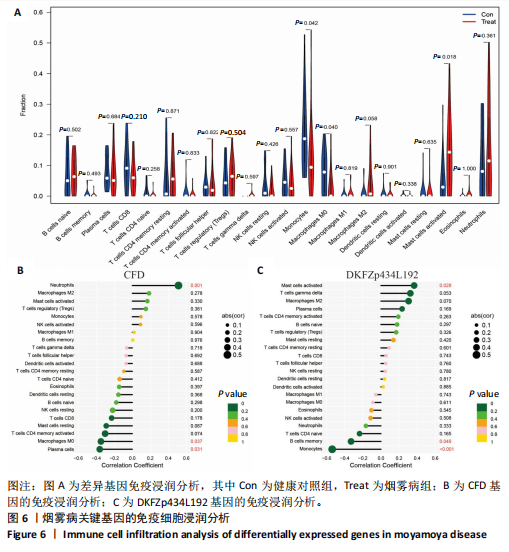
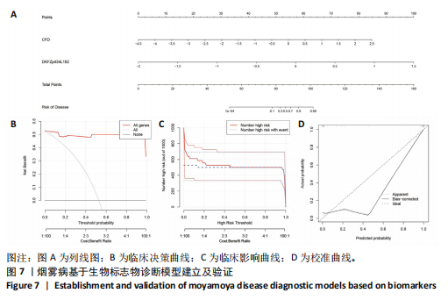
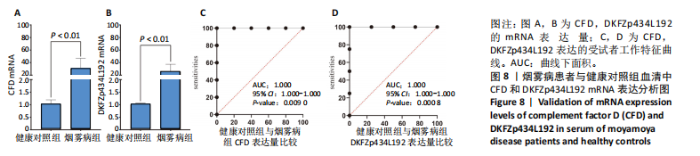
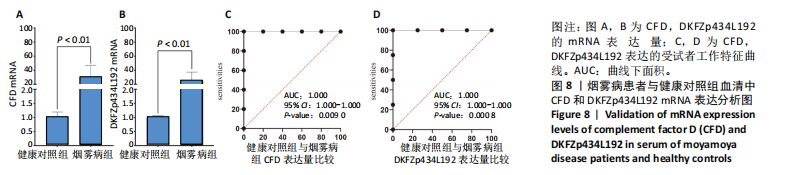
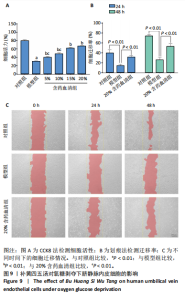
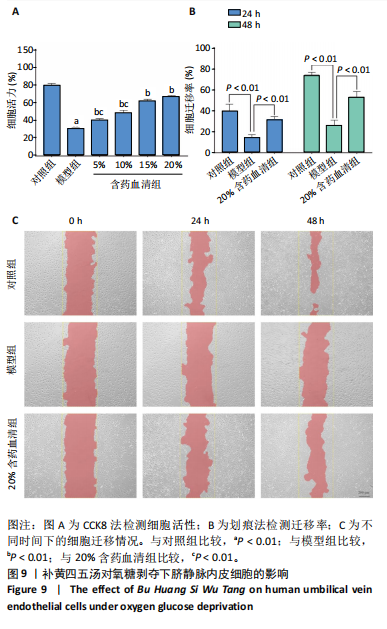
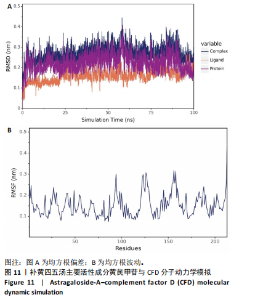
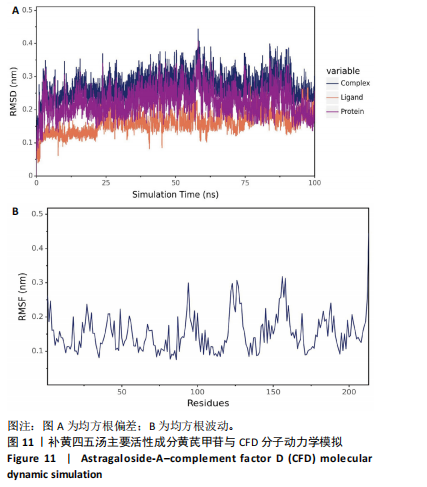
2.1 烟雾病差异表达基因鉴定 使用R语言中的“sva”包,将2个数据集GSE157628和GSE144025合并,创建训练集。使用“limma”包,设置筛选条件为|logFC|> 1且P < 0.05,从训练集中识别出76个差异基因,其中49个上调、27个下调。图1展示了这些差异基因的热图和火山图。 2.2 烟雾病差异基因富集分析 此次研究对76个候选基因进行GO功能注释,挖掘各个基因所代表的生物学意义,结果筛选条件为P < 0.05。GO分析包括3个类别,即生物过程、细胞成分及分子功能。GO富集分析显示,差异基因在生物过程中显著富集于神经嵴细胞分化与迁移、干细胞发育与分化、细胞基质黏附的调节等过程;显著富集的细胞成分有:含胶原蛋白的细胞外基质、阳离子通道复合体、神经递质受体复合体等成分;在分子功能上显著富集于:跨膜受体蛋白激酶活性、细胞因子及生长因子结合、血清素受体活性等功能,如图2A。DO富集分析的主要途径是阿米巴样细胞迁移、细胞基质黏附、间质细胞分化与迁移等,如图2B。对候选基因进行KEGG功能富集分析,筛选条件为P < 0.05,结果显示KEGG富集分析的主要途径包括补体途径、hh信号通路、金黄色葡萄球菌感染等,如图2C。Metascape富集分析的主要途径还包括氨基多糖代谢、冠脉发育、血管内皮生长因子刺激下的细胞反应,如图2D。为了探究76个候选基因在蛋白质水平的相互作用,将它们上传至STRING数据库(https://string-db.org/)进行蛋白互作分析,阈值设置为综合得分≥0.40,结果显示CFD主要与CEBPA、KIT、ALDH1A1、GAB1、SOX10和GPC3等基因相关,如图2E。 2.3 烟雾病诊断标志物的筛选与验证 LASSO(Least absolute shrinkage and selection operator)分析是一种数据挖掘方法,在多元线性回归中添加惩罚系数,通过不断压缩系数从而对数据进行降维及模型精简,有效避免了共线性和过拟合。基于上一步PH假定检验的结果,使用R包“glmnet”对候选预后基因进行LASSO回归分析进一步筛选。与疾病重要性较强的基因压缩较小,而重要性较弱的基因压缩成0,进行10折交叉验证后得到了LASSO回归常见的有2个图形,一个是基因系数的图形(图3A),一个是交叉验证的误差图(图3B),利用 LASSO 共识别出16个有意义的特征基因。在训练集的全部样本中,基于候选基因,使用“e1071”包进行支持向量机递归特征消除分析,k-fold交叉验证设置k=10,halve above=50,获取每个基因的重要性及重要性排名,同时得到每次迭代组合的错误率和准确率,选择错误率最低点为最佳组合。图3C,D显示了不同数量变量对模型预测误差的影响。在此次研究中,当最优变量数量为8个时,模型的预测误差最低为0.025,得到8个基因。2种算法交集出6个重叠基因,如图3E,分别为CFD、DKFZp434L192、EN1、MIA、MYOT和OGN,这些基因在烟雾病组的表达与对照组相比均具有显著差异,如图4A,其曲线下面积分别为 0.873,0.873,0.839,0.895,0.898和0.930,表明上述6种基因有作为烟雾病诊断生物标志物的可能性,如图4B。利用GSE189993数据集对上述6种诊断生物标志物进行验证,结果显示CFD和DKFZp434L192在烟雾病组显著上调(P < 0.01,P < 0.05),如图4C。利用受试者工作特征曲线测试上述6种生物标志物的诊断效能,结果显示CFD和DKFZp434L192的曲线下面积分别为 0.831和0.732,说明以上2种标志物具有明确的诊断价值,如图4D。单基因富集分析结果显示,CFD主要富集在中性粒细胞胞外陷阱形成、Th17细胞分化、弓形虫感染、EB病毒感染、趋化因子信号转导等通路,如图5A;DKFZp434L192主要富集在肿瘤坏死因子信号通路、军团菌感染、疱疹病毒感染、白细胞介素17信号通路等通路,如图5B。 2.4 烟雾病关键基因的免疫细胞浸润分析 与健康对照组相比,烟雾病组活化肥大细胞的细胞丰度显著升高(P < 0.05),单核细胞、MO巨噬细胞的细胞丰度显著下降(P < 0.05),如图6A。根据相关性分析结果,CFD与中性粒细胞的升高以及MO巨噬细胞、浆细胞的降低表现出显著的相关性(P < 0.05),如图6B;DKFZp434L192与活化肥大细胞的升高以及记忆B细胞、单核细胞的降低表现出显著的相关性(P < 0.05),如图6C。 2.5 烟雾病预测列线图的构建与评价 基于R软件“rms”包构建了烟雾病的诊断列线图,如图7A;决策曲线显示CFD+DKFZp434L192的曲线远高于灰色线,说明诊断模型具有较高精准度,如图7B;根据决策曲线计算出临床影响曲线,高风险曲线(红色)与蓝色表示的真阳性患者曲线非常接近,见图7C;校准曲线中粗实线代表的高风险曲线,与粗虚线代表的真阳性患者曲线非常接近,如图7D,说明烟雾病的得病风险与预测风险之间的误差较小,进一步表明列线图模型对烟雾病预测的高准确性。 2.6 qRT-PCR验证烟雾病生物标志物 与健康对照组相比,烟雾病组CFD mRNA表达明显上调(P < 0.01),如图8A,DKFZp434L192 mRNA表达明显上调(P < 0.01),如图8B,进一步验证了该2个生物标志物的诊断效能。当将烟雾病组与健康对照组进行比较时,CFD表达的曲线下面为1(95%CI:1.000-1.000,P=0.009),见图8C,DKFZp434L192表达的曲线下面积为1(95%CI:1.000-1.000,P=0.000 8),见图8D。 2.7 CCK-8法检测人脐静脉内皮细胞活性实验结果 如图9A所示,模型组细胞活性显著低于对照组(P < 0. 01),不同浓度含药血清组细胞活性显著高于模型组(P < 0.01),并表现出浓度依赖性,说明补黄四五汤能显著提升氧糖剥夺下的人脐静脉内皮细胞增殖活性。 2.8 人脐静脉内皮细胞划痕迁移实验结果 3组细胞均随着时间变化进行了不同程度的迁移,相同时间下,对照组细胞迁移率最高,模型组细胞迁移率最低,见图9B,C,说明补黄四五汤能显著提高氧糖剥夺下的人脐静脉内皮细胞迁移能力。 2.9 补黄四五汤主要成分与目标靶点CFD分子对接结果 结果显示,补黄四五汤的4种主要活性成分均能与CFD蛋白对接,活性成分和关键靶点间的相互作用可视化见图10。黄芪甲苷、黄芪多糖、刺芒柄花素、毛蕊异黄酮与目标靶点CFD的对接结合能分别为-36.0,-20.3,-30.2,-28.9 kJ/mol,均低于-20.934 kJ/mol,表明上述4种活性成分与CFD亲合度较高,其中黄芪甲苷与CFD蛋白的结合能最低,该活性成分和CFD蛋白亲和度可能最高且最稳定。 2.10 补黄四五汤主要活性成分与单倍复合物的分子动力学模拟结果 复合物结构的均方根偏差高于蛋白和配体,并且随模拟的进行逐渐稳定,未发生剧烈波动,表明复合物结构逐渐稳定,如图11A。模拟过程中均方根波动较低,如图11B所示,表明残基较为稳定。复合物中的范德华力相互作用能ΔEvdw[-179.269±5.82) kJ/mol]显著高于静电相互作用ΔEele[(-47.462±4.112) kJ/mol],前者是后者的3.8倍,同时这两者的能量均高于疏水相互作用ΔEnonpol[(-24.616±0.579) kJ/mol]。因此,在结合能的组成中,范德华力相互作用发挥主要作用,静电相互作用发挥次要作用,疏水相互作用发挥补充作用。小分子与蛋白的ΔEMMPBSA=(-85.768±2.672) kJ/mol(ΔEMMPBSA=ΔEele+ΔEvdw+ΔEpol+ΔEnonpol),二者结合能与亲和力较高。"

| [1] FUJIMURA M, TOMINAGA T, KURODA S, et al. 2021 Japanese Guidelines for the Management of Moyamoya Disease: Guidelines from the Research Committee on Moyamoya Disease and Japan Stroke Society. Neurol Med Chir (Tokyo). 2022;62(4):165-170. [2] KURODA S, FUJIMURA M, TAKAHASHI J, et al. Diagnostic Criteria for Moyamoya Disease - 2021 Revised Version. Neurol Med Chir (Tokyo). 2022;62(7):307-312. [3] KURODA S, HOUKIN K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008;7(11):1056-1066. [4] UCHINO K, JOHNSTON SC, BECKER KJ, et al. Moyamoya disease in Washington State and California. Neurology. 2005;65(6):956-958. [5] KIM JS. Moyamoya Disease: Epidemiology, Clinical Features, and Diagnosis. J Stroke. 2016;18(1):2-11. [6] GONZALEZ NR, AMIN-HANJANI S, BANG OY, et al. Adult Moyamoya Disease and Syndrome: Current Perspectives and Future Directions: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke. 2023;54(10):e465-e479. [7] LI J, JIN M, SUN X, et al. Imaging of Moyamoya Disease and Moyamoya Syndrome: Current Status. J Comput Assist Tomogr. 2019;43(2): 257-263. [8] YANG H, HUANG G, LI X, et al. High-resolution magnetic resonance vessel wall imaging provides new insights into Moyamoya disease. Front Neurosci. 2024;18:1375645. [9] 中华医学会神经外科学分会.烟雾病和烟雾综合征诊断与治疗中国专家共识(2024版)[J].中华神经外科杂志,2024,40(3):220-229. [10] PORRAS JL, YANG W, XU R, et al. Effectiveness of Ipsilateral Stroke Prevention Between Conservative Management and Indirect Revascularization for Moyamoya Disease in a North American Cohort. World Neurosurg. 2018;110:e928-e936. [11] SMITH ER, SCOTT RM. Surgical management of moyamoya syndrome. Skull Base. 2005;15(1):15-26. [12] RAMESH V, BERNARDI B, STAFA A, et al. Intracerebral large artery disease in Aicardi-Goutières syndrome implicates SAMHD1 in vascular homeostasis. Dev Med Child Neurol. 2010;52(8):725-732. [13] KIM T, OH CW, BANG JS, et al. Moyamoya Disease: Treatment and Outcomes. J Stroke. 2016;18(1):21-30. [14] BAO XY, DUAN L. Chinese expert consensus on the treatment of MMD. Chin Neurosurg J. 2023;9(1):5. [15] 杨培中.EDAS联合通窍益髓汤治疗缺血型烟雾病的临床疗效分析[D].济南:山东中医药大学,2017. [16] 王馨慧,王苏童,吕穆杰,等.黄芪桂枝五物汤调节M1/M2巨噬细胞改善炎症反应的分子机制研究[J]. 北京中医药大学学报,2023, 46(6):801-810.
[17] YUN W, DAN W, LIU J, et al. Investigation of the Mechanism of Traditional Chinese Medicines in Angiogenesis through Network Pharmacology and Data Mining. Evid Based Complement Alternat Med. 2021;2021:5539970. [18] VANCURA J, BOYD NK, VOGEL BN, et al. Rapidly progressive moyamoya vasculopathy stabilized with immunotherapy in aicardi-goutières syndrome. J Neurol. 2024;271(2):1019-1022. [19] HE S, ZHOU Z, ZHANG J, et al. Differential WNT4 expression among various subtypes of moyamoya disease results in alterations of microtubule stability. Clin Transl Med. 2024;14(8):e1797. [20] JIN F, DUAN C. Identification of immune-infiltrated hub genes as potential biomarkers of Moyamoya disease by bioinformatics analysis. Orphanet J Rare Dis. 2022;17(1):80. [21] ZHOU Z, WANG Y, ZHANG J, et al. Characterization of PANoptosis-related genes and the immune landscape in moyamoya disease. Sci Rep. 2024;14(1):10278. [22] HAN Z, ZHANG J, SU Y, et al. Identification of oxidative phosphorylation-related genes in moyamoya disease by combining bulk RNA-sequencing analysis and machine learning. Front Genet. 2024;15:1417329. [23] LI W, ZHAO X, FU J, et al. Identification of lysosome-related hub genes as potential biomarkers and immune infiltrations of moyamoya disease by multiple bioinformatics methods and machine-learning strategies. Heliyon. 2024;10(14):e34432. [24] LI J, HE Q, ZHENG Z, et al. Comprehensive Analysis and In Vitro Verification of Endothelial-Mesenchymal Transition-Related Genes in Moyamoya Disease. Mol Neurobiol. 2024. doi: 10.1007/s12035-024-04423-x. [25] XU Y, CHEN B, GUO Z, et al. Identification of diagnostic markers for moyamoya disease by combining bulk RNA-sequencing analysis and machine learning. Sci Rep. 2024;14(1):5931. [26] Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the CoW, Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo). 2012;52(5):245-266. [27] KAMADA F, AOKI Y, NARISAWA A, et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J Hum Genet. 2011;56(1):34-40. [28] LI J, HE Q, LIU C, et al. Integrated analysis of the association between methionine cycle and risk of moyamoya disease. CNS Neurosci Ther. 2023;29(11):3212-3227. [29] HE S, ZHANG J, LIU Z, et al. Upregulated Cytoskeletal Proteins Promote Pathological Angiogenesis in Moyamoya Disease. Stroke. 2023;54(12): 3153-3164. [30] YIN Z, GE P, ZENG C, et al. Association of lysine pathway metabolites with moyamoya disease. Clin Nutr. 2024;43(3):787-795. [31] GÓMEZ-BANOY N, GUSEH JS, LI G, et al. Adipsin preserves beta cells in diabetic mice and associates with protection from type 2 diabetes in humans. Nat Med. 2019;25(11):1739-1747. [32] ABHINAV K, LEE AG, PENDHARKAR AV, et al. Comprehensive Profiling of Secreted Factors in the Cerebrospinal Fluid of Moyamoya Disease Patients. Transl Stroke Res. 2024;15(2):399-408. [33] CHEN X, SONG C, MA X, et al. High lipoprotein(a) concentration is associated with moyamoya disease. Lipids Health Dis. 2024;23(1):21. [34] DEB-CHATTERJI M, KELLER CW, KOCH S, et al. Profiling Complement System Components in Primary CNS Vasculitis. Cells. 2021;10(5):1139. [35] VOLANAKIS JE, NARAYANA SV. Complement factor D, a novel serine protease. Protein Sci. 1996;5(4):553-564. [36] MULLARD A. FDA approves first complement factor D inhibitor. Nat Rev Drug Discov. 2024;23(5):329. [37] GU SX, YAROVINSKY TO, HWA J. Fishing for “complements” with vascular organoid models of microvascular disease. Cell Stem Cell. 2023;30(10):1285-1286. [38] JIN S, REESINK KD, KROON AA, et al. Complement factors D and C3 cross-sectionally associate with arterial stiffness, but not independently of metabolic risk factors: The Maastricht Study. J Hypertens. 2022; 40(11):2161-2170. [39] KAWAKAMI E, SAIKI N, YONEYAMA Y, et al. Complement factor D targeting protects endotheliopathy in organoid and monkey models of COVID-19. Cell Stem Cell. 2023;30(10):1315-1330.e10. [40] ITO S, HASHIMOTO H, YAMAKAWA H, et al. The complement C3-complement factor D-C3a receptor signalling axis regulates cardiac remodelling in right ventricular failure. Nat Commun. 2022;13(1):5409. [41] RICKLIN D, REIS ES, LAMBRIS JD. Complement in disease: a defence system turning offensive. Nat Rev Nephrol. 2016;12(7):383-401. [42] KATO M, KUDO Y, HATASE M, et al. Moyamoya Disease Associated with a Deficiency of Complement Component 6. J Stroke Cerebrovasc Dis. 2022;31(8):106601. [43] LIU XM, RUAN XZ, CAI Z, et al. Moyamoya disease caused by leptospiral cerebral arteritis. Chin Med J (Engl). 1980;93(9):599-604. [44] SUZUKI J, KODAMA N. Moyamoya disease--a review. Stroke. 1983;14(1): 104-109. [45] TAKAYANAGI K, KANAMORI F, ISHII K, et al. Higher abundance of Campylobacter in the oral microbiome of Japanese patients with moyamoya disease. Sci Rep. 2023;13(1):18545. [46] GE P, TAO C, WANG W, et al. Circulating immune cell landscape and T-cell abnormalities in patients with moyamoya disease. Clin Transl Med. 2024;14(4):e1647. [47] KIM JH, JEON H, KIM M, et al. Chemical and perfusion markers as predictors of moyamoya disease progression and complication types. Sci Rep. 2024;14(1):56. [48] LI H, CAO X, GU X, et al. GM-CSF Promotes the Development of Dysfunctional Vascular Networks in Moyamoya Disease. Neurosci Bull. 2024;40(4):451-465. [49] LIU C, GE P, ZHANG B, et al. Mass cytometry revealed the circulating immune cell landscape across different Suzuki stages of Moyamoya disease. Immunol Res. 2024;72(4):654-664. [50] WU J, LU AD, ZHANG LP, et al. Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi. 2019;40(1):52-57. [51] GORLA G, CARROZZINI T, POLLACI G, et al. Increase of Circulating Endothelial Progenitor Cells and Released Angiogenic Factors in Children with Moyamoya Arteriopathy. Int J Mol Sci. 2023;24(2):1233. [52] 陈宝田.陈宝田教授经方临床应用[M].广州:广东科技出版社, 2014. [53] 黄仕营, 谢炜.陈宝田教授时方临床应用[M].北京:科学出版社, 2017. [54] 骆文婷,陈泽伟,伍志勇,等.补黄四五汤治疗周围性面瘫的疗效及安全性[J].暨南大学学报(自然科学与医学版),2018,39(4):332-338. [55] BERSANO A, GUEY S, BEDINI G, et al. Research Progresses in Understanding the Pathophysiology of Moyamoya Disease. Cerebrovasc Dis. 2016;41(3-4):105-118. [56] BEDINI G, BLECHARZ KG, NAVA S, et al. Vasculogenic and Angiogenic Pathways in Moyamoya Disease. Curr Med Chem. 2016;23(4):315-345. |
| [1] | Yan Laijun, Ge Haiya, Wang Zhengming, Yang Zongrui, Niu Lifeng, Zhan Hongsheng. Mechanism by which Tongdu Huoxue Decoction inhibits macrophage inflammation to delay intervertebral disc degeneration in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6851-6857. |
| [2] | Nigeayi · Aihemaiti, Yilidanna · Dilixiati, An Wei, Maimaitituxun · Tuerdi. Expression of mitochondrial creatine kinase 2 in a rat model of temporomandibular joint osteoarthritis and its role in inflammation progression [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6877-6884. |
| [3] | Wang Ziheng, Wu Shuang. Oxidative stress-related genes and molecular mechanisms after spinal cord injury: data analysis and verification based on GEO database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6893-6904. |
| [4] | Zhao Xuemei, Wang Rui, Ao · Wuliji, Bao Shuyin, Jiang Xiaohua. Effects of Agiophyllum Oligo Saccharides on inflammation and apoptosis of mouse synovial cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6939-6946. |
| [5] | Zhu Jiaping, Gao Bo, Lou Chunbiao, Yang Fengyong, Yang Kun. Monomeric traditional Chinese medicine in the treatment of rheumatoid arthritis: regulation of T cell balance [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6955-6962. |
| [6] | Yao Tingfeng, Liu Lin, Liu Shixuan, Lu Xinyue. Meta-analysis of the effectiveness of dry needling at myofascial trigger points in the treatment of knee disorders [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6989-6996. |
| [7] | Wu Zhenhua, Zhang Xiwei, Wang Yipin, Li Qianqian. Relationship between seven serum lipid traits and osteoarthritis: a large sample analysis of European population in IEU OPEN GWAS database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 7004-7014. |
| [8] | Chen Hui, Zhang Lu, Chen Jing, Wang Nanzhang, Wang Ruochun, Lu Cuihua, Ji Yifei. Screening of target genes for bile acid metabolism in Crohn’s disease and its value in disease diagnosis and therapeutic monitoring [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 7028-7040. |
| [9] | Lu Xiuli, Xu Huazhen, Chen Yuxing, Yao Nan, Hu Zixuan, Huang Dane. Mechanism of Jiangu Formula in treating osteoporosis based on osteoclast-osteoblast coupling [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6828-6835. |
| [10] | Yu Qinghe, Cai Ziming, Wu Jintao, Ma Pengfei, Zhang Xin, Zhou Longqian, Wang Yakun, Lin Xiaoqin, Lin Wenping. Vanillic acid inhibits inflammatory response and extracellular matrix degradation of endplate chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6391-9397. |
| [11] | Fan Jiaxin, Jia Xiang, Xu Tianjie, Liu Kainan, Guo Xiaoling, Zhang Hui, Wang Qian . Metformin inhibits ferroptosis and improves cartilage damage in osteoarthritis model rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6398-6408. |
| [12] | Zhou Ying, Tian Yong, Zhong Zhimei, Gu Yongxiang, Fang Hao. Inhibition of tumor necrosis factor receptor associated factor 6 regulates mTORC1/ULK1 signaling and promotes autophagy to improve myocardial injury in sepsis mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6434-6440. |
| [13] | Wang Wanchun, , Yi Jun, Yan Zhangren, Yang Yue, Dong Degang, Li Yumei. 717 Jiedu Decoction remodels homeostasis of extracellular matrix and promotes repair of local injured tissues in rats after Agkistrodon halys bite [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6457-6465. |
| [14] | Zhang Xin, Guo Baojuan, Xu Huixin, Shen Yuzhen, Yang Xiaofan, Yang Xufang, Chen Pei. Protective effects and mechanisms of 3-N-butylphthalide in Parkinson’s disease cell models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6466-6473. |
| [15] | Zhang Songjiang, Li Longyang, Zhou Chunguang, Gao Jianfeng. Central anti-inflammatory effect and mechanism of tea polyphenols in exercise fatigue model mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6474-6481. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||