Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (26): 5652-5661.doi: 10.12307/2025.725
Previous Articles Next Articles
Intestinal flora regulates bone metabolism: a visual analysis of literature from the Web of Science Core Collection
Zeng Hao1, Zou Shunyi1, Li Zhengpeng1, Chai Yuan1, 2, Huang Yourong1, 2, Zhang Xiaoyun1, 2
- 1Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China; 2Department of Orthopedics, Ruikang Hospital, Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China
-
Received:2024-09-09Accepted:2024-10-18Online:2025-09-18Published:2025-02-27 -
Contact:Zhang Xiaoyun, MD, Associate chief physician, Master’s supervisor, Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China; Department of Orthopedics, Ruikang Hospital, Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China -
About author:Zeng Hao, Master candidate, Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China -
Supported by:National Natural Science Foundation of China, No. 82360937 (to ZXY); Natural Science Foundation of Guangxi Zhuang Autonomous Region (General Program), No. 2023GXNSFAA026075 (to ZXY); Guangxi Traditional Chinese Medicine Appropriate Technology Development and Promotion Project, No. GZSY22-36 (to ZXY); Huang Yourong GUI School of Traditional Chinese Medicine Master Training Project, No. [2022]6 (to HYR); Innovation Project of Guangxi Graduate Education, No. YCSY2023038 (to ZH)
CLC Number:
Cite this article
Zeng Hao, Zou Shunyi, Li Zhengpeng, Chai Yuan, Huang Yourong, Zhang Xiaoyun. Intestinal flora regulates bone metabolism: a visual analysis of literature from the Web of Science Core Collection[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5652-5661.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
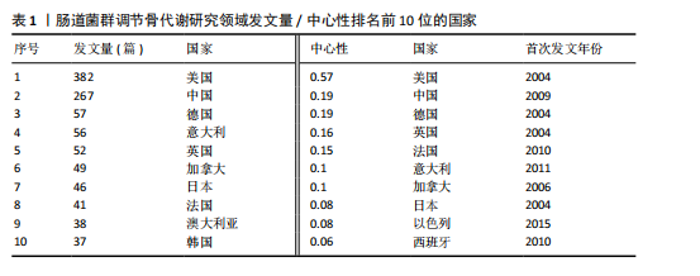
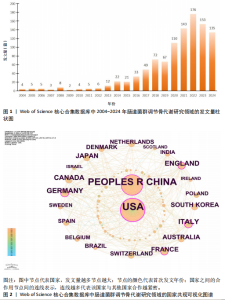
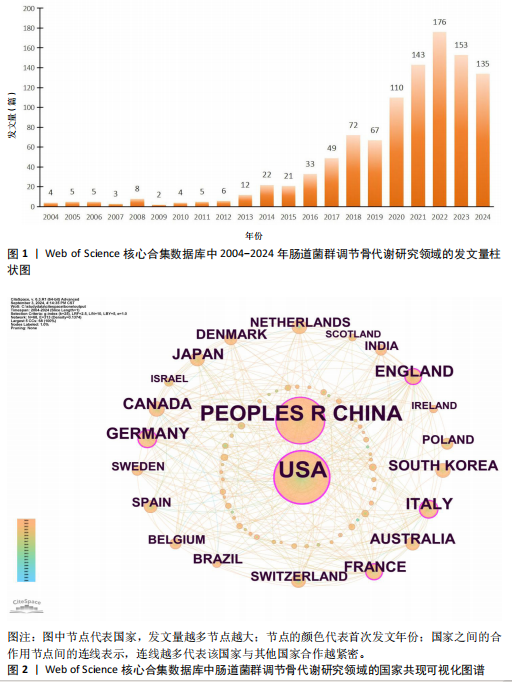
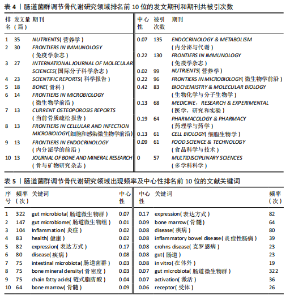
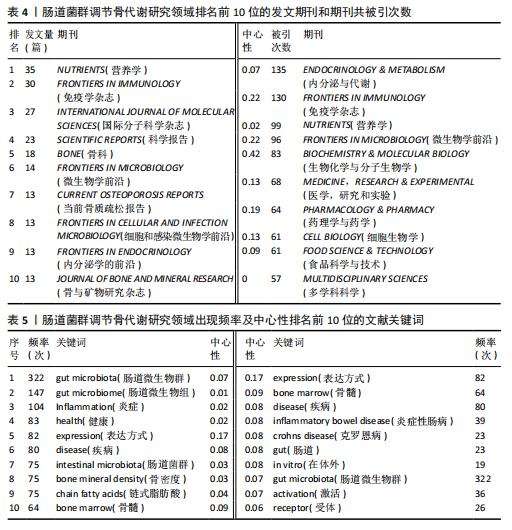
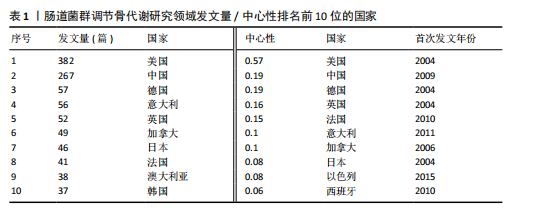
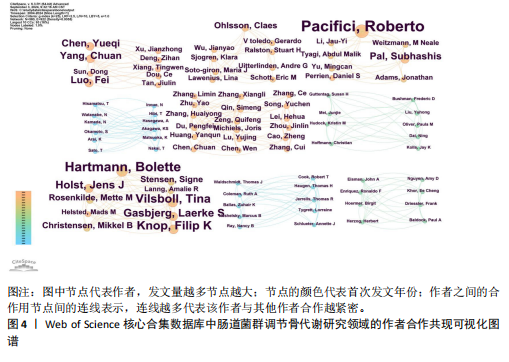
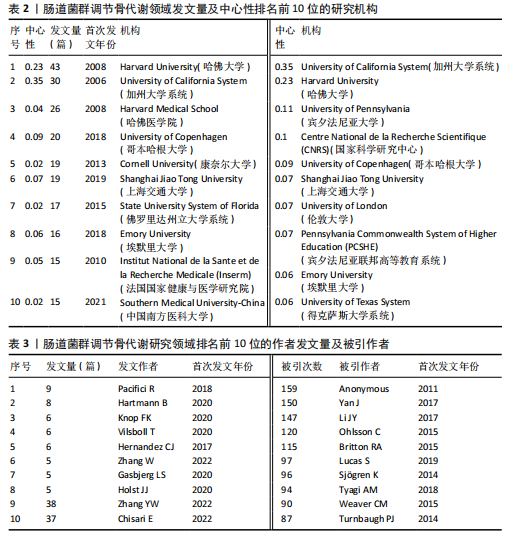
2.1 发文量分析 数据统计显示,发文量最少的年份为2009年(2篇),最多的年份为2022年(176篇)。从2004-2012年,发文量一直保持在较低水平,从2013年开始,发文量出现显著的增长,特别是从2017年开始,每年的发文量都超过了40篇,并在接下来的几年里持续增长;到了2022年,发文量达到了176篇,是之前年份发文量的数倍;2023年和2024年的发文量也继续保持在高位,分别为153篇和134篇,这表明肠道菌群调节骨代谢领域的研究活动在不断增加,可能受到了更多的关注和投入,但需要注意的是,虽然发文量在不断增长,但不同年份之间的增长速度和幅度存在差异,这可能与该领域的研究热点、环境、资金等多种因素相关,见图1。 2.2 发文国家分析 通过对文献的发文国家共现分析发现,近20年间肠道菌群调节骨代谢研究领域发文量前10位的国家分别为美国、中国、德国、意大利、英国、加拿大、日本、法国、澳大利亚和韩国。美国自2004年起共发文382篇,中国自2009年起共发文267篇,可以看出美国和中国在该领域的研究及出版活动中占据了主导地位。虽然中国的首次发文年份比美国较晚,但发文数量已经接近美国,这表明中国在该领域的研究逐渐增多。德国、意大利、英国、加拿大、日本、法国、澳大利亚和韩国等国家也在该领域做出了重要贡献,表明肠道菌群调节骨代谢的研究已经跨越国界,成为全球性的研究热点。此外,网络中心性最高的是美国(0.57),其次是中国(0.19)。综合发文量、发文年份以及中心性,美国是该领域研究最为突出的国家,其他国家虽然发文数量较少,但也可能在特定研究领域上有所专长或贡献,随着时间的推移,这些国家的发文数量也可能会有所增加,见表1,图2。 2.3 发文机构分析 通过对文献的发文机构共现分析发现,发文量前10位的机构分别为哈佛大学(43篇)、加州大学系统(30篇)、哈佛医学院(26篇)、哥本哈根大学(20篇)、康奈尔大学(19篇)、上海交通大学(19篇)、佛罗里达州立大学系统(17篇)、埃默里大学(16篇)、法国国家健康与医学研究院(15篇)、中国南方医科大学(15篇)。研究中心性最高的分别是加州大学系统(0.35)和哈佛大学(0.23),均表现出了较强的研究实力,并且形成了十分紧密的学术合作关系网。综合分析,无论是哈佛大学、加州大学系统等老牌强校,还是上海交通大学、中国南方医科大学等新兴力量,都在特定年份内展示了较强的研究实力,而加州大学系统和哈佛大学是肠道菌群调节骨代谢领域的主要研究机构,这些机构不仅发文量高,而且研究质量也较高,并逐渐形成较为紧密的学术合作关系网。虽然其他机构的发文量尚可,但机构间合作较少,加强科学研究的合作交流更有利于研究的深入,从而有利于推动科学研究的发展。见表2,图3。 2.4 发文作者和作者共被引分析 通过对近20年间肠道菌群调节骨代谢研究领域相关文献的发文作者共现分析发现,纳入的1 035篇文献由774位作者发表,作者间共有1 747次合作。经Citespace软件处理,得到共现可视化图谱,见图4。其中发文量最高的为埃默里大学的Pacifici R教授,自2018年起共发表了9篇文献,此外,Hartmann B、Knop FK、Vilsboll T等作者之间的合作网络也较为突出,为近年来该领域内较为活跃的研究者,均自2020年起发表了6篇及以上的文献。此外,发表的文献被引用次数最多的作者是Anonymous(159次),其次是Yan J(150次)和Li JY(147次),说明他们的研究具有重要影响力,为多数学者所认可,见表3。 2.5 发文期刊和期刊共被引分析 对纳入的文献进行数据统计和分析可得,该领域研究文献共发表在788种期刊上。其中《NUTRIENTS》期刊收录文献最多(35篇),被引用次数最高的期刊为《ENDOCRINOLOGY & METABOLISM》(135次),但其中心性仅为0.07,而《BIOCHEMISTRY & MOLECULAR BIOLOGY》期刊中心性"

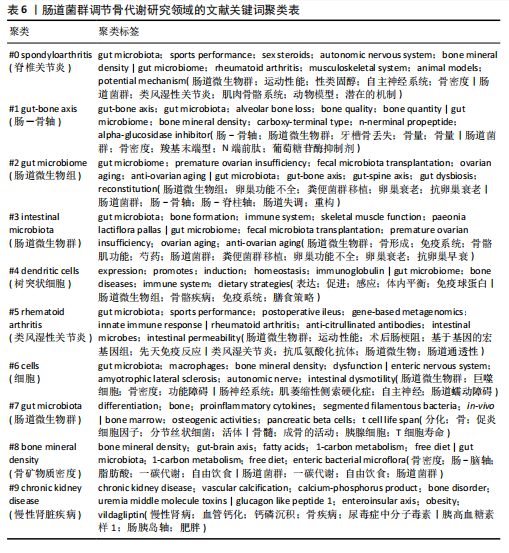
最高为0.42,《IMMUNOLOGY》和《FRONTIERS IN MICROBIOLOGY》期刊发文量和中心性均有较好表现,表明文献质量被众多研究者认可,是该领域中较有影响力的期刊,见表4。 2.6 关键词分析 2.6.1 关键词共现分析 在文献中,关键词扮演着至关重要的角色,它们不仅是文献内容的高度概括和精炼表达,还是连接文献与读者、研究者和数据库检索系统的桥梁,而通过对纳入文献的关键词进行分析,可以更好地把握肠道菌群调节骨代谢领域的研究热点。通过软件对文献的关键词共现分析,得到关键词共现图谱,见图5。数据分析共得到文献关键词572个,共现频率最高的关键词为gut microbiota(322次),其次是gut microbiome(147次)和inflammation(104次),中心性最高的关键词是expression(0.17),其次是bone marrow(0.09)和disease(0.08),见表5。 综合关键词出现频次和中心性,并排除与此研究主题直接相关的关键词,可知该领域的核心关键词是:inflammatory bowel disease(炎症性肠病)、crohns-disease(克罗恩病)、chain fatty acids(链式脂肪酸)、bone marrow(骨髓)。 2.6.2 关键词聚类分析 对文献的关键词进行聚类分析并绘制图谱,聚类以聚类模块值(Q)和平均轮廓值(S)作为聚类可信度和真实性的评判标准。一般认为,聚类模块值(Q) > 0.3可以认为聚类结构显著,平均轮廓值(S) > 0.7可以认为聚类是高效率且有说服力的。该研究基于关键词共现聚类,得出聚类模块值(Q)=0.802 7,平均轮廓值(S)=0.914 6,说明该研究聚类结构显著,聚类具有可信度。选取排名前10的聚类,见图6。聚类#0反映了肠道菌群在脊椎关节炎方面的研究;聚类#1反映了肠道菌群在牙槽骨丢失中的相关研究;聚类#2、聚类#3反映了肠道菌群与绝经后骨质疏松症的相关研究;聚类#4反映了树突状细胞在肠道菌群调节骨代谢疾病方面的研究;聚类#5反映了肠道菌群在类风湿性关节炎方面的研究;聚类#6反映了巨噬细胞在肠道菌群调节骨代谢疾病方面的研究;聚"
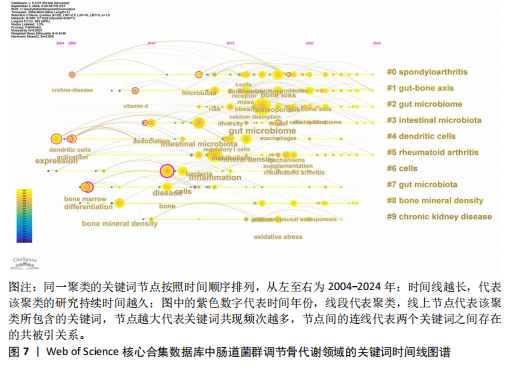
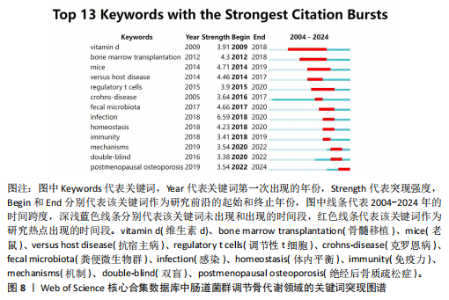
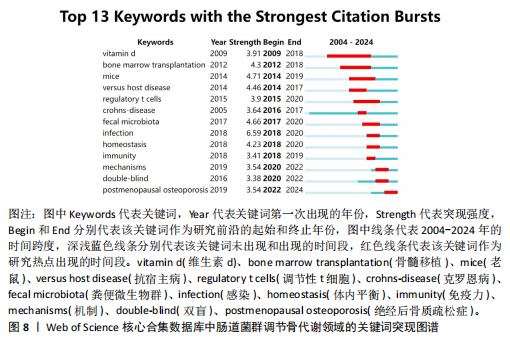
类#7反映了促炎细胞因子在肠道菌群调节骨代谢方面的研究;聚类#8反映了肠-脑轴在肠道菌群调节骨代谢方面的研究;聚类#9反映了肠道菌群在慢性肾脏疾病方面的研究,见表6。 2.6.3 关键词时间线分析 使用软件对肠道菌群调节骨代谢研究领域的文献关键词按时间线进行聚类分析,基于聚类的时间线可以清楚地观察不同聚类的时间跨度,见图7。如图所示,crohns-disease(克罗恩病)作为一种肠道炎症性疾病,与#0 spondyloarthritis (脊椎关节炎)的关系被广泛研究,时间跨度从2005年延续至2024年,是持续时间最长的聚类,说明该聚类标签已形成稳定的研究方向,是该领域研究的持续热点。#0 spondyloarthritis(脊椎关节炎)和#5 rheumatoid Arthritis(类风湿性关节炎)作为两种具有代表性的关节炎,可能是该领域的研究起点疾病。此外,根据关键词聚类分析和关键词时间线图谱可以明确该领域的研究方向及趋势,postmenopausal osteoporosis (绝经后骨质疏松症)、rheumatoid arthritis(类风湿性关节炎)、oxidative stress(氧化应激)和mitochondrial dysfunction(线粒体功能障碍)为该领域的最新研究前沿。 2.6.4 关键词突现分析 突现分析主要关注某一时间段内某个关键词引用量的显著变化,可以反映出这一时间段研究者对肠道菌群调节骨代谢领域的关注侧重点,见图8。如图所示,bone marrow transplantation(骨髓移植)的被引用量从2012年开始急剧上升,在2018年达到峰值,crohns-disease(克罗恩病)虽然在2005年就开始有相关研究,但真正的突现是在2016-2017年,这可能表明不同时间段在以上两项研究领域取得了显著的研究进展或突破而备受关注,如单倍体相合造血干细胞移植技术的突破、首次提出克罗恩病患者的肠道炎症和营养吸收障碍可诱发骨质疏松症。homeostasis(稳态)、immunity(免疫)和mechanisms(机制)这些关键词分别在2018-2020年、2018-2019年和2019-2022年间显示了引用强度的突现,反映了该领域在"

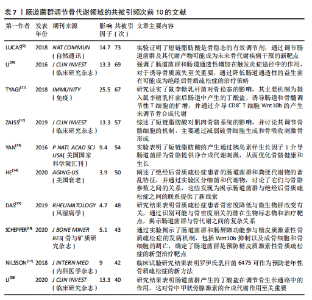
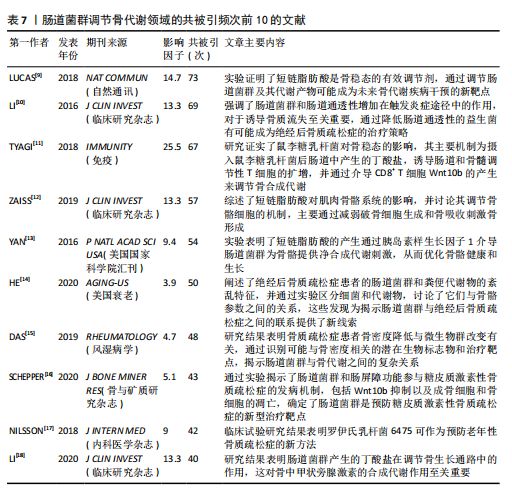
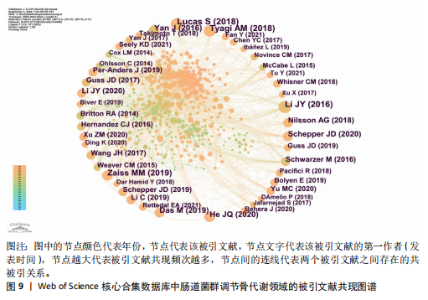
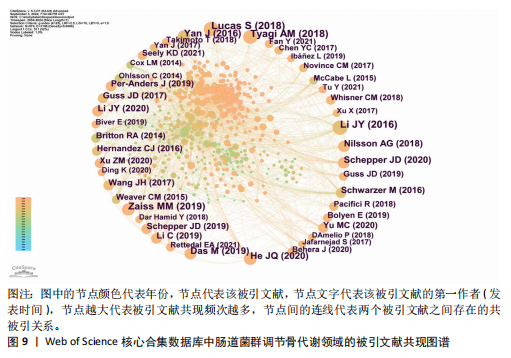
不同时间段内对特定生物过程和机制进行了深入研究。postmenopausal osteoporosis(绝经后骨质疏松症)是图中较晚出现且持续时间较长的突现关键词,表明对绝经后骨质疏松症的研究在未来可能还会持续增加。 2.7 文献共被引分析 文献共被引分析是一种在文献计量学和科学研究中常用的方法,它通过分析文献之间的共同引用关系来揭示学科领域内的知识结构、研究热点和趋势,其共被引次数越高说明该文献具有学术价值[8]。对纳入文献进行共被引分析,得到974个节点,3 108条连线,密度为0.066的被引文献共现图谱,见图9。在被引频次排名前10的文献中,4篇文献探讨了短链脂肪酸影响骨代谢的机制;3篇文献探讨了肠道菌群分别在绝经后骨质疏松症、糖皮质激素性骨质疏松症和老年性骨质疏松症中的发病机制及治疗潜力;1篇文献探讨了肠道炎症与肠道菌群、甲状旁腺激素和骨代谢的关联性;1篇文献探讨了鼠李糖乳杆菌、T细胞对肠道菌群和骨代谢的影响。具体统计和总结,见表7。"
| [1] SRIVASTAVA RK, SAPRA L, MISHRA PK. Osteometabolism: Metabolic Alterations in Bone Pathologies. Cells. 2022;11(23):3943. [2] ZHOU M, AN YZ, GUO Q, et al. Energy homeostasis in the bone. Trends Endocrinol Metab. 2024;35(5):439-451. [3] FU L, ZHANG P, WANG Y, et al. Microbiota-bone axis in ageing-related bone diseases. Front Endocrinol (Lausanne). 2024;15: 1414350. [4] INCHINGOLO AM, GARGIULO ISACCO C, INCHINGOLO AD, et al. The human microbiota key role in the bone metabolism activity. Eur Rev Med Pharmacol Sci. 2023; 27(6):2659-2670. [5] BEHERA J, ISON J, TYAGI SC, et al. The role of gut microbiota in bone homeostasis. Bone. 2020;135:115317. [6] LI C, PI G, LI F. The Role of Intestinal Flora in the Regulation of Bone Homeostasis. Front Cell Infect Microbiol. 2021;11:579323. [7] NINKOV A, FRANK JR, MAGGIO LA. Bibliometrics: Methods for studying academic publishing. Perspect Med Educ. 2022;11(3):173-176. [8] HASSAN W, DUARTE AE. Bibliometric analysis: A few suggestions. Curr Probl Cardiol. 2024;49(8):102640. [9] LUCAS S, OMATA Y, HOFMANN J, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9(1):55. [10] LI JY, CHASSAING B, TYAGI AM, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049-2063. [11] TYAGI AM, YU M, DARBY TM, et al. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity. 2018;49(6):1116-1131.e7. [12] ZAISS MM, JONES RM, SCHETT G, et al. The gut-bone axis: how bacterial metabolites bridge the distance. J Clin Invest. 2019; 129(8):3018-3028. [13] YAN J, HERZOG JW, TSANG K, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016;113(47):E7554-E7563. [14] HE J, XU S, ZHANG B, et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging (Albany NY). 2020; 12(9):8583-8604. [15] DAS M, CRONIN O, KEOHANE DM, et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology (Oxford). 2019; 58(12):2295-2304. [16] SCHEPPER JD, COLLINS F, RIOS-ARCE ND, et al. Involvement of the Gut Microbiota and Barrier Function in Glucocorticoid-Induced Osteoporosis. J Bone Miner Res. 2020;35(4):801-820. [17] NILSSON AG, SUNDH D, BÄCKHED F, et al. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018;284(3):307-317. [18] LI JY, YU M, PAL S, et al. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J Clin Invest. 2020;130(4):1767-1781. [19] D’AMELIO P, SASSI F. Gut Microbiota, Immune System, and Bone. Calcif Tissue Int. 2018;102(4):415-425. [20] GUO M, LIU H, YU Y, et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes. 2023;15(1):2190304. [21] ZHOU B, YUAN Y, ZHANG S, et al. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front Immunol. 2020;11:575. [22] ZHANG YW, SONG PR, WANG SC, et al. Diets intervene osteoporosis via gut-bone axis. Gut Microbes. 2024;16(1):2295432. [23] ADAK A, KHAN MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76(3):473-493. [24] WEI H, ZHAO Y, XIANG L. Bone health in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2023;17(9):921-935. [25] CUSHING K, HIGGINS PDR. Management of Crohn Disease: A Review. JAMA. 2021; 325(1):69-80. [26] KE K, ARRA M, ABU-AMER Y. Mechanisms Underlying Bone Loss Associated with Gut Inflammation. Int J Mol Sci. 2019;20(24): 6323. [27] AL-DAGHRI NM, AZIZ I, YAKOUT S, et al. Inflammation as a contributing factor among postmenopausal Saudi women with osteoporosis. Medicine (Baltimore). 2017;96(4):e5780. [28] MONTALVANY-ANTONUCCI CC, DUFFLES LF, DE ARRUDA JAA, et al. Short-chain fatty acids and FFAR2 as suppressors of bone resorption. Bone. 2019;125:112-121. [29] WALLIMANN A, MAGRATH W, THOMPSON K, et al. Gut microbial-derived short-chain fatty acids and bone: a potential role in fracture healing. Eur Cell Mater. 2021;41:454-470. [30] BAO M, ZHANG K, WEI Y, et al. Therapeutic potentials and modulatory mechanisms of fatty acids in bone. Cell Prolif. 2020;53(2): e12735. [31] LIU X, ZHANG H, SHI G, et al. The impact of gut microbial signals on hematopoietic stem cells and the bone marrow microenvironment. Front Immunol. 2024; 15:1338178. [32] LORENZO J. From the gut to bone: connecting the gut microbiota with Th17 T lymphocytes and postmenopausal osteoporosis. J Clin Invest. 2021;131(5): e146619. [33] XU Q, LI D, CHEN J, et al. Crosstalk between the gut microbiota and postmenopausal osteoporosis: Mechanisms and applications. Int Immunopharmacol. 2022;110:108998. [34] GUAN Z, XUANQI Z, ZHU J, et al. Estrogen deficiency induces bone loss through the gut microbiota. Pharmacol Res. 2023;196: 106930. [35] FENG R, WANG Q, YU T, et al. Quercetin ameliorates bone loss in OVX rats by modulating the intestinal flora-SCFAs-inflammatory signaling axis. Int Immunopharmacol. 2024;136:112341. [36] ZHANG YW, CAO MM, LI YJ, et al. Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J Orthop Translat. 2022;37:46-60. [37] ZHAO T, WEI Y, ZHU Y, et al. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front Immunol. 2022;13: 1007165. [38] LIN L, ZHANG K, XIONG Q, et al. Gut microbiota in pre-clinical rheumatoid arthritis: From pathogenesis to preventing progression. J Autoimmun. 2023;141:103001. [39] CAMPBELL C, KANDALGAONKAR MR, GOLONKA RM, et al. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines. 2023;11(2):294. [40] LI J, JIA N, CUI M, et al. The intestinal mucosal barrier - A key player in rheumatoid arthritis? Clin Anat. 2023;36(7):977-985. [41] KIMBALL JS, JOHNSON JP, CARLSON DA. Oxidative Stress and Osteoporosis. J Bone Joint Surg Am. 2021;103(15):1451-1461. [42] RIEGGER J, SCHOPPA A, RUTHS L, et al. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review. Cell Mol Biol Lett. 2023;28(1):76. [43] SHANDILYA S, KUMAR S, KUMAR JHA N, et al. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J Adv Res. 2021;38: 223-244. [44] ZONG Y, LI H, LIAO P, et al. Mitochondrial dysfunction: mechanisms and advances in therapy. Signal Transduct Target Ther. 2024;9(1):124. [45] KOWALCZYK P, SULEJCZAK D, KLECZKOWSKA P, et al. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int J Mol Sci. 2021; 22(24):13384. [46] SUN K, JING X, GUO J, et al. Mitophagy in degenerative joint diseases. Autophagy. 2021;17(9):2082-2092. [47] AILIOAIE LM, AILIOAIE C, LITSCHER G. Gut Microbiota and Mitochondria: Health and Pathophysiological Aspects of Long COVID. Int J Mol Sci. 2023;24(24):17198. |
| [1] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [2] | Liang Haobo, Wang Zeyu, Ma Wenlong, Liu Hao, Liu Youwen. Hot issues in the field of joint revision: infection, rehabilitation nursing, bone defect, and prosthesis loosening [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1963-1971. |
| [3] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [4] | Lyu Liting, Yu Xia, Zhang Jinmei, Gao Qiaojing, Liu Renfan, Li Meng, Wang Lu. Bibliometric analysis of research process and current situation of brain aging and exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1457-1465. |
| [5] | Xie Liugang, Cui Shuke, Guo Nannan, Li Aoyu, Zhang Jingrui. Research hotspots and frontiers of stem cells for Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1475-1485. |
| [6] | Chang Jinxia, Liu Yufei, Niu Shaohui, Wang Chang, Cao Jianchun. Visualization analysis of macrophage polarization in tissue repair process [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1486-1496. |
| [7] |
Zhao Wensheng, Li Xiaolin, Peng Changhua, Deng Jia, Sheng Hao, Chen Hongwei, Zhang Chaoju, He Chuan.
Gut microbiota and osteoporotic fractures #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1296-1304.
|
| [8] | Li Huijun, Li Huangyan, Zhang Yeting. Physical activity and cognition in older adults: research hotspot and topic evolution [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1073-1080. |
| [9] |
Sun Guanghan, Xie Zhencong, Sun Mi, Xu Yang, Guo Dong.
Therapeutic effect and mechanism by which Trichosanthis Fructus-Allii Macrostemonis Bulbus regulates gut microbiota in a rat model of coronary heart disease #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 917-927.
|
| [10] | Dang Xiaowen, Huang Hailiang, Huang Lei, Wang Yajie . Research frontiers and hotspots of carbon nanomaterials in biomedical field over the past 10 years [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 752-760. |
| [11] | Ma Yucong, Ouyang Zhengzheng, Liu Xiaojie, Yang Sifei. Tracking of research trends and hotspots in medical magnesium alloy materials [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7470-7480. |
| [12] | Wang Yong, Li Hongyu, Liu Yuhang, Wang Fengxing. Knowledge map of surgical treatment for osteonecrosis of the femoral head: a bibliometric analysis of data from 2005 to 2024 [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(33): 7250-7260. |
| [13] | Guo Haizhen, Cong Zidong, Zhao Yuke, Li Xiaofeng, Yu Lu, Qian Shule, Wang Runying, Du Wuxun. Development of patch clamp technology in the past 10 years: visual analysis based on CiteSpace and VOSviewer [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6717-6726. |
| [14] | Cui Yuena, Chen Xiaoyu, Liang Meiting, Chen Wujin, He Yi, Dilinur·Ekpa, Du Manxi, Zhu Yuqiu, Abuduwupuer·Haibier, Sun Yuping. Differences of calorie restriction and time-restricted feeding on metabolic indices and gut microbiota of mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6449-6456. |
| [15] | Zhao Xiaoxuan, Liu Shuaiyi, Xing Zheng, Li Qingwen, Chu Xiaolei, Li Qi. Research hotspots and trends in application of tissue engineering in peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6591-6600. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||