Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (24): 5237-5244.doi: 10.12307/2025.723
Previous Articles Next Articles
Effects of voluntary exercise on molecular expression profiles in the hippocampus of mice: a gene expression profile analysis based on the GEO database
Ye Xing1, 2, Liu Renyi2
- 1School of Sports and Human Sciences, Beijing Sport University, Beijing 100084, China; 2School of Physical Education, China University of Geosciences (Wuhan), Wuhan 430074, Hubei Province, China
-
Received:2024-07-31Accepted:2024-10-08Online:2025-08-28Published:2025-02-06 -
Contact:Liu Renyi, PhD, Professor, School of Physical Education, China University of Geosciences (Wuhan), Wuhan 430074, Hubei Province, China -
About author:Ye Xing, Doctoral candidate, School of Sports and Human Sciences, Beijing Sport University, Beijing 100084, China; School of Physical Education, China University of Geosciences (Wuhan), Wuhan 430074, Hubei Province, China -
Supported by:the “Outstanding Talent Cultivation Fund” of the Fundamental Research Funds for the Central Universities, No. CUG150607 (to LRY)
CLC Number:
Cite this article
Ye Xing, Liu Renyi. Effects of voluntary exercise on molecular expression profiles in the hippocampus of mice: a gene expression profile analysis based on the GEO database[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5237-5244.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
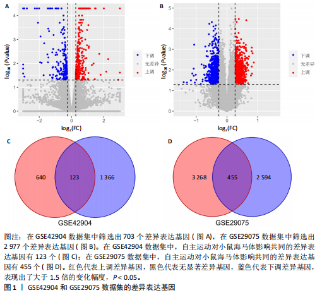
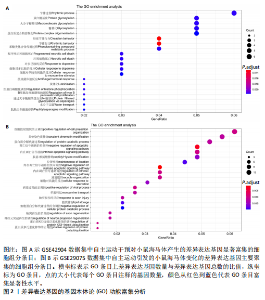
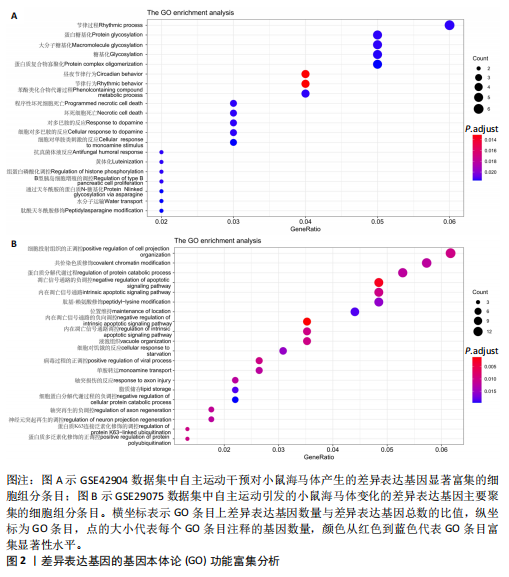
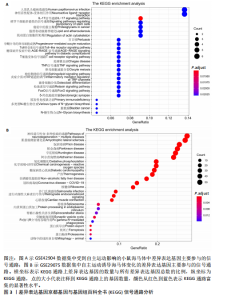
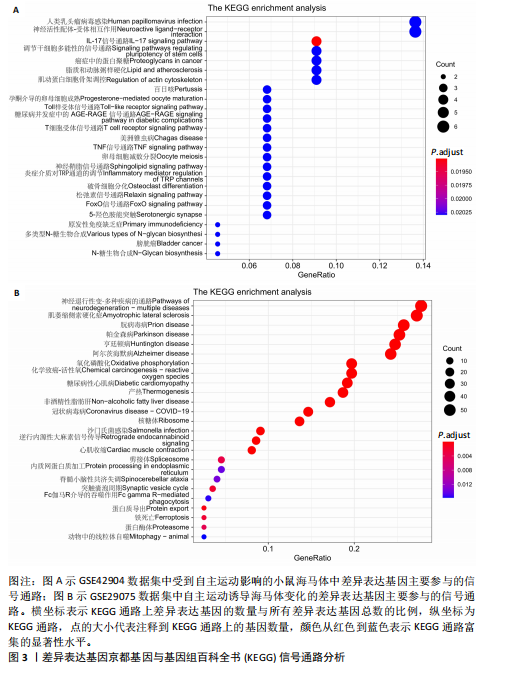
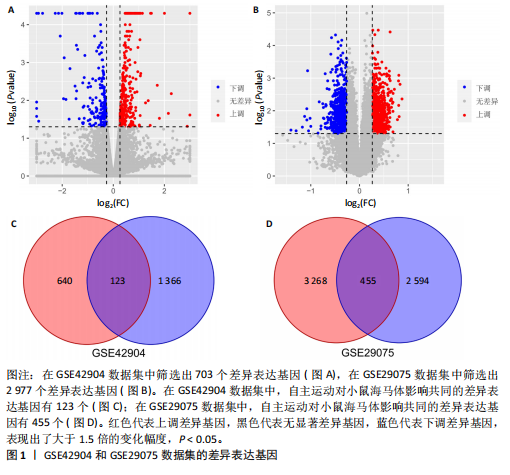
2.1 微阵列数据信息与差异表达基因筛选 采用R语言的Limma包对标准化数据进行基因筛选,筛选条件设定为|log2FC| > 1.5且p-adjust < 0.05。在GSE42904数据集中筛选出703个差异表达基因(图1A),在GSE29075数据集中筛选出2 977个差异表达基因(图1B)。此次研究采用重叠分析法对2套微阵列数据集进行统计分析,结果显示:在GSE42904数据集中,自主运动对小鼠海马体影响共同的差异表达基因有123个(图1C);在GSE29075数据集中,自主运动对小鼠海马体影响共同的差异表达基因有455个(图1D)。 2.2 差异表达基因的基因本体论功能富集分析 GSE42904数据集中,自主运动干预对小鼠海马体产生的差异表达基因显著富集在节律过程(Rhythmic process)、糖基化(Glycosylation)、蛋白复合物寡聚化(Protein complex oligomerization)、坏死性细胞死亡(Necrotic cell death)等生物学过程;受体配体活性(Receptor ligand activity)、ATP酶活性(ATPase activity)、磷酸酶结合(Phosphatase binding)等分子功能;分泌颗粒(Secretory granule)、细胞顶端部分(Apical part of cell)、顶端质膜(Apical plasma membrane)、神经元致密核心囊泡(Neuronal dense core vesicle)等细胞组分条目,见图2A。 GSE29075数据集中,自主运动引发的差异表达基因主要聚集在蛋白质分解代谢过程的调控(Regulation of protein catabolic process)、细胞投射组织正调控(Positive regulation of cell projection organization)、共价染色质修饰(Covalent chromatin modification)、凋亡负调控(Negative regulation of apoptotic)等生物学过程;泛素蛋白质连接酶结合(Ubiquitin protein ligase binding)、N-甲基转移酶活性(N-methyltransferase activity)、蛋白-赖氨酸(Protein-lysine)等分子功能;溶酶体(Lysosome)、内体膜(Endosome membrane)、甲基转移酶复合物(Methyltransferase complex)等细胞组分条目,见图2B。 2.3 差异表达基因的KEGG通路分析 GSE42904数据集中,通过对受到自主运动影响的小鼠海马体中差异表达基因进行KEGG通路分析,结果显示,差异表达基因主要参与人类乳头状瘤病毒感染(Human papillomavirus infection)、神经活性配体-受体相互作用(Neuroactive ligand-receptor interaction)、白细胞介素17信号通路(IL-17 signaling pathway)、钙信号通路(Calcium signaling pathway)、Toll样受体信号通路(Toll-like receptor signaling pathway)、乙醇信号通路(Alcoholism)、磷脂酰肌醇-3-激酶-Akt信号通路(PI3K-Akt signaling pathway)等,见图3A。GSE29075数据集中,自主运动诱导的海马体变化的差异表达基因主要参与神经退行性疾病-多种疾病通路(Pathways of neurodegeneration- multiple diseases)、肌萎缩性侧束硬化(Amyotrophic lateral sclerosis)、阿尔茨海默病(Alzheimer diseases)、朊病毒病(Prion disease)、帕金森病(Parkinson disease)、亨廷顿病(Huntington disease)、环磷酸腺苷信号通路(cAMP signaling pathway)等信号通路,见图3B。 2.4 差异表达基因的蛋白-蛋白互作网络构建和模块分析 利用STRING在线数据库为筛选出的差异表达基因构建蛋白-蛋白互作网络。在GSE42904数据集中,针对自主运动"
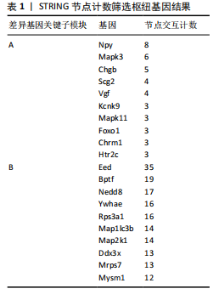
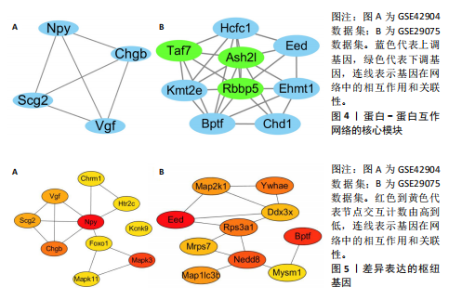
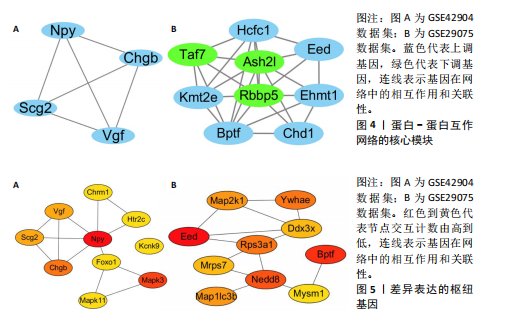
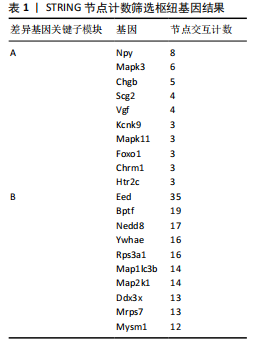
对小鼠海马体影响的差异表达基因,所构建的蛋白-蛋白互作网络包含53个节点和46条边。在Cytoscape软件中,通过使用MCODE插件,识别出了网络中的核心模块的基因,共鉴定出3个重要的中心模块。其中,第一个模块的基因包括神经肽Y(Neuropeptide Y,Npy)、VGF神经生长因子诱导(VGF nerve growth factor inducible,Vgf)、嗜铬粒蛋白B (Chromogranin B,Chgb)和分泌颗粒素Ⅱ(secretogranin Ⅱ,Scg2),且均为上调基因(图4A)。GSE29075数据集中,自主运动干预对小鼠海马体影响的差异表达基因,分析了差异表达基因的蛋白-蛋白互作网络,该网络包含428条边和213个节点,共鉴定出7个中心模块。在第1个模块中,上调基因包括解旋酶DNA结合蛋白1 (Chromodomain helicase DNA binding protein 1,Chd1)、赖氨酸(K)特异性甲基转移酶2E(lysine (K)-specific methyltransferase 2E,Kmt2e)、胚胎外胚层发育(embryonic ectoderm development,Eed)、宿主细胞因子C1 (Host cell factor C1,Hcfc1)、全色组蛋白甲基转移酶1(Euchromatic histone methyltransferase 1,Ehmt1)、溴域PHD指转录因子(Bromodomain PHD finger transcription factor,Bptf),下调基因包括视网膜母细胞瘤结合蛋白5组蛋白赖氨酸甲基转移酶复合体亚基(Retinoblastoma binding protein 5,histone lysine methyltransferase complex subunit,Rbbp5)、TATA盒结合蛋白相关因子7(TATA-box binding protein associated factor 7,Taf7)、ASH2样组蛋白赖氨酸甲基转移酶复合体亚基(ASH2 like histone lysine methyltransferase complex subunit,Ash2l),见图4B。 2.5 枢纽基因筛选 使用Cytoscape软件中的cytohubba插件,采用“Degree”算法确定差异表达基因中排名前10的枢纽基因,见表1。GSE42904数据集中,自主运动对小鼠海马影响的枢纽基因包括Npy、Mapk3、Chgb、Scg2、Vgf、Kcnk9、Mapk11、Foxo1、Chrm1和Htr2c(图5A)。GSE29075数据集中,自主运动对年龄诱导的海马体变化的枢纽基因包括Eed、Bptf、Nedd8、Ywhae、Rps3a1、"
| [1] PALAUS M, MARRON EM, VIEJO-SOBERA R, et al. Neural Basis of Video Gaming: A Systematic Review. Front Hum Neurosci. 2017;11:248. [2] PLUVINAGE JV, WYSS-CORAY T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci. 2020;21(2):93-102. [3] VAN STRIEN NM, CAPPAERT NL, WITTER MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10(4):272-282. [4] WYSS-CORAY T, MUCKE L. Inflammation in neurodegenerative disease-a double-edged sword. Neuron. 2002;35(3):419-432. [5] ZHANG YM, DAI QF, CHEN WH, et al. Effects of acupuncture on cortical expression of Wnt3a, β-catenin and Sox2 in a rat model of traumatic brain injury. Acupunct Med. 2016;34(1):48-54. [6] BRAKSIEK M, THORMANN TF, WICKER P. Intentions of Environmentally Friendly Behavior Among Sports Club Members: An Empirical Test of the Theory of Planned Behavior Across Genders and Sports. Front Sports Act Living. 2021;3:657183. [7] XU X, JERSKEY BA, COTE DM, et al. Cerebrovascular perfusion among older adults is moderated by strength training and gender. Neurosci Lett. 2014;560:26-30. [8] BOLANDZADEH N, TAM R, HANDY TC, et al. Resistance Training and White Matter Lesion Progression in Older Women: Exploratory Analysis of a 12-Month Randomized Controlled Trial. J Am Geriatr Soc. 2015; 63(10):2052-2060. [9] KENNEY WL, WILMORE JH, COSTILL DL. Physiology of sport and exercise. Human Kinetics, 2022. [10] FORTE R, TOCCI N, DE VITO G. The Impact of Exercise Intervention with Rhythmic Auditory Stimulation to Improve Gait and Mobility in Parkinson Disease: An Umbrella Review. Brain Sci. 2021;11(6):685. [11] DOS SANTOS JR, BORTOLANZA M, FERRARI GD, et al. One-Week High-Intensity Interval Training Increases Hippocampal Plasticity and Mitochondrial Content without Changes in Redox State. Antioxidants (Basel). 2020;9(5):445. [12] CHO JW, JUNG SY, LEE SW, et al. Treadmill exercise ameliorates social isolation-induced depression through neuronal generation in rat pups. J Exerc Rehabil. 2017;13(6):627-633. [13] JIANG H, CHEN S, WANG L, et al. An Investigation of Limbs Exercise as a Treatment in Improving the Psychomotor Speed in Older Adults with Mild Cognitive Impairment. Brain Sci. 2019;9(10):277. [14] VOSS MW, NAGAMATSU LS, LIU-AMBROSE T, et al. Exercise, brain, and cognition across the life span. J Appl Physiol (1985). 2011;111(5):1505-1513. [15] MA Q. Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci Bull. 2008;24(4):265-270. [16] ANG ET, GOMEZ-PINILLA F. Potential therapeutic effects of exercise to the brain. Curr Med Chem. 2007;14(24):2564-2571. [17] NAYLOR AS, BULL C, NILSSON MK, et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008;105(38):14632-14637. [18] NOTA MH, DOHM-HANSEN S, NICOLAS S, et al. A cafeteria diet blunts effects of exercise on adult hippocampal neurogenesis but not neurogenesis-dependent behaviours in adult male rats. BioRxiv. 2024: 2024.07. 16.603714. [19] SEUDRE O, CARRILLO-BALTODANO AM, LIANG Y, et al. ERK1/2 is an ancestral organising signal in spiral cleavage. Nat Commun. 2022;13(1):2286. [20] PATHAN M, KEERTHIKUMAR S, ANG CS, et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics. 2015; 15(15):2597-2601. [21] TERVO DG, HWANG BY, VISWANATHAN S, et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron. 2016;92(2):372-382. [22] GAO Y, SYED M, ZHAO X. Mechanisms underlying the effect of voluntary running on adult hippocampal neurogenesis. Hippocampus. 2023;33(4):373-390. [23] RAO YL, GANARAJA B, MURLIMANJU BV, et al. Hippocampus and its involvement in Alzheimer’s disease: a review. 3 Biotech. 2022;12(2):55. [24] VIVAR C, PETERSON BD, VAN PRAAG H. Running rewires the neuronal network of adult-born dentate granule cells. Neuroimage. 2016;131:29-41. [25] ZHU G, FANG Y, CUI X, et al. Magnolol upregulates CHRM1 to attenuate Amyloid-β-triggered neuronal injury through regulating the cAMP/PKA/CREB pathway. J Nat Med. 2022;76(1):188-199. [26] LIU Z, GAO W, XU Y. Eleutheroside E alleviates cerebral ischemia-reperfusion injury in a 5-hydroxytryptamine receptor 2C (Htr2c)-dependent manner in rats. Bioengineered. 2022;13(5):11718-11731. [27] YOU JC, MURALIDHARAN K, PARK JW, et al. Epigenetic suppression of hippocampal calbindin-D28k by ΔFosB drives seizure-related cognitive deficits. Nat Med. 2017; 23(11):1377-1383. [28] HANON O, PEQUIGNOT R, SEUX ML, et al. Relationship between antihypertensive drug therapy and cognitive function in elderly hypertensive patients with memory complaints. J Hypertens. 2006;24(10):2101-2107. [29] BARHWAL K, HOTA S K, BAITHARU I, et al. Isradipine antagonizes hypobaric hypoxia induced CA1 damage and memory impairment: Complementary roles of L-type calcium channel and NMDA receptors. Neurobiol Dis. 2009;34(2):230-244. [30] CLAPHAM DE. Calcium signaling. Cell. 2007; 131(6):1047-58. [31] MALIK BR, GILLESPIE JM, HODGE JJ. CASK and CaMKII function in the mushroom body α’/β’ neurons during Drosophila memory formation. Front Neural Circuits. 2013;7:52. [32] NA KS, JUNG HY, KIM YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. [33] BEN-ARI S, OFEK K, BARBASH S, et al. Similar cation channels mediate protection from cerebellar exitotoxicity by exercise and inheritance. J Cell Mol Med. 2012;16(3):555-568. [34] WAN C, SHI L, LAI Y, et al. Long-term voluntary running improves cognitive ability in developing mice by modulating the cholinergic system, antioxidant ability, and BDNF/PI3K/Akt/CREB pathway. Neurosci Lett. 2024;836:137872. [35] CHISHOLM SP, CERVI AL, NAGPAL S, et al. Interleukin-17A increases neurite outgrowth from adult postganglionic sympathetic neurons. J Neurosci. 2012;32(4):1146-1155. [36] PEARSON JRD, REGAD T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct Target Ther. 2017;2:17040. [37] DRAGO A, SERRETTI A. Focus on HTR2C: A possible suggestion for genetic studies of complex disorders. Am J Med Genet B Neuropsychiatr Genet. 2009;150b(5):601-637. [38] LIU F, LIANG C, LI Z, et al. Human iPSC-derived neuron of 16p11. 2 deletion reveals haplotype-specific expression of MAPK3 and its contribution to variable NDD phenotypes. BioRxiv. 2022: 2022.07.10.498576. [39] GEIL CR, HAYES DM, MCCLAIN JA, et al. Alcohol and adult hippocampal neurogenesis: promiscuous drug, wanton effects. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:103-113. [40] BIJAK M. Neuropeptide Y reduces epileptiform discharges and excitatory synaptic transmission in rat frontal cortex in vitro. Neurosci. 2000;96(3):487-494. [41] DECRESSAC M, WRIGHT B, DAVID B, et al. Exogenous neuropeptide Y promotes in vivo hippocampal neurogenesis. Hippocampus. 2011;21(3):233-238. [42] MALVA JO, XAPELLI S, BAPTISTA S, et al. Multifaces of neuropeptide Y in the brain--neuroprotection, neurogenesis and neuroinflammation. Neuropeptides. 2012; 46(6):299-308. [43] STILLMAN CM, ESTEBAN-CORNEJO I, BROWN B, et al. Effects of Exercise on Brain and Cognition Across Age Groups and Health States. Trends Neurosci. 2020;43(7): 533-543. [44] DI BENEDETTO G, IANNUCCI LF, SURDO NC, et al. Compartmentalized signaling in aging and neurodegeneration. Cells-Basel. 2021;10(2):464. [45] DEN HEIJER T, VAN DER LIJN F, KOUDSTAAL PJ, et al. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain. 2010;133(Pt 4):1163-1172. [46] MAASS A, DÜZEL S, GOERKE M, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2015;20(5):585-593. [47] ROSJAT N, LIU L, WANG BA, et al. Aging-associated changes of movement-related functional connectivity in the human brain. Neuropsychologia. 2018;117: 520-529. [48] SPEISMAN RB, KUMAR A, RANI A, et al. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun. 2013;28: 25-43. [49] VIVAR C, PETERSON B, PINTO A, et al. Running throughout Middle-Age Keeps Old Adult-Born Neurons Wired. eNeuro. 2023; 10(5):ENEURO.0084-23.2023. [50] ZHANG Y, XU H. Identifying an APP-binding protein in neuronal cell death. Molecular Neurodegeneration. 2012;7(Suppl 1):L22. [51] WANG YY, DENG YS, DAI SK, et al. Loss of microglial EED impairs synapse density, learning, and memory. Mol Psychiatry. 2022;27(7):2999-3009. [52] BAHRAMPOUR S, JONSSON C, THOR S. Brain expansion promoted by polycomb-mediated anterior enhancement of a neural stem cell proliferation program. PLoS Biol. 2019;17(2):e3000163. [53] KUEHNER JN, YAO B. The Dynamic Partnership of Polycomb and Trithorax in Brain Development and Diseases. Epigenomes. 2019;3(3):17-24. |
| [1] | Ma Hong, Ding Xueling, Wang Qi, Lyu Hui, Asya Albusm, Cheng Xinyi, Ma Xiang. Expression and significance of tumor necrosis factor alpha, nuclear factor kappaB and ionized calcium binding adaptor molecule-1 in the hippocampus of mice with aortic dissection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 858-863. |
| [2] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [3] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [4] | Lei Qi, Zhao Bingbing, Luo Hong, Chen Qiang, Jiang Yan. Influenza A virus recombinant hemagglutinin 1 induces the production of beta-defensin and interferon-gamma in mouse tracheal epithelial cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6905-6912. |
| [5] | Zhao Xuemei, Wang Rui, Ao · Wuliji, Bao Shuyin, Jiang Xiaohua. Effects of Agiophyllum Oligo Saccharides on inflammation and apoptosis of mouse synovial cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6939-6946. |
| [6] | Deng Qing, Wang Qingjun, Zhang Yeting. Visual analysis of dynamic evolution of research topics in the field of physical activity and hippocampal tissue [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6997-7003. |
| [7] | Zhao Nan, Ding Yong, Xiu Hang, Liu Pengfei, Liang Guogang. Extraction and culture of enteric glial cells from C57BL/6 newborn neonatal mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6656-6660. |
| [8] | Luo Dan, Ge Zhilin, Hou Yonghui, Wang Wanshun, Zhan Jiheng, Hou Yu, Lin Dingkun, Chen Shudong. Extraction and subculture of neural stem cells from mouse embryonic spinal cord: comparison and analysis on advantages and disadvantages of three commonly used digestive enzymes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6609-6615. |
| [9] | Chen Lijuan, Gao Xinxue, Wu Jin, Du Ying, Lyu Meijun, Sui Guoyuan, Jia Lianqun, Pan Guowei. Construction and evaluation of spleen-deficiency hyperlipidemia mouse models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6237-6242. |
| [10] | Wu Xiaochou, Wang Huiying, Wang Jie, Zhang Caifeng, Hou Yanyun, Jin Bo. Protective mechanism of tanshinone IIA in mouse ovarian cryopreservation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6198-6204. |
| [11] | Chen Ying, Liu Jian, Liang Yajie, Li Yanqing, Song Lijuan, Huang Jianjun, Yu Jiezhong, Wang Qing, Ma Cungen . Mechanism by which hydroxysafflor yellow A alleviates demyelination in cuprizone mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5311-5319. |
| [12] | Liu Kexin, Ma Chao, Liu Kai, Hao Maochen, Wang Xingru, Meng Lingting, Dong Mei, Wang Jianzhong . Screening and validation of glucose metabolism genes in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4181-4189. |
| [13] | Lin Meiyu, Yao Xiang, Gao Jing, Zhao Xilong, Pan Xinghua, Ruan Guangping. Comparison of biological characteristics of adipose-derived stem cells in young and old mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4063-4068. |
| [14] | Jiang Qiang, Yu Jie, Geng Zixiang, Wang Ning, Guo Jia, Yang Guangyue, Wang Peige, Zhao Yongfang. Comparison of phenotypes and mechanistic characteristics in two mouse models of sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 2922-2929. |
| [15] | Yang Kun, Zhang Rong, Wu Yue, Lei Xiaoping, Shen Yunchuan, Kang Lan, Dong Wenbin. Construction of a mouse model for alveolar type II epithelial cell-specific knockout of SENP1 gene based on the Cre-loxP recombinase system [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 2943-2950. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||