Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (20): 4190-4204.doi: 10.12307/2025.682
Previous Articles Next Articles
Anti-osteoporotic mechanisms of kaempferol based on gut microbiota and comprehensive targeted metabolomics
Liang Zhou1, 2, Zhang Chi1, Pan Chengzhen2, Yang Bo2, Pu Zhanglin1, Liu Hua1, Peng Jinhui2, Wen Lichun2, Ling Guanhan2, Chen Feng1
- 1Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China; 2Yulin Integrated Traditional Chinese and Western Medicine Orthopedic Hospital, Yulin 537599, Guangxi Zhuang Autonomous Region, China
-
Received:2024-07-12Accepted:2024-08-13Online:2025-07-18Published:2024-12-19 -
Contact:Chen Feng, MD, Chief physician, Professor, Doctoral supervisor, Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China -
About author:Liang Zhou, MD candidate, Associate chief physician, Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China; Yulin Integrated Traditional Chinese and Western Medicine Orthopedic Hospital, Yulin 537599, Guangxi Zhuang Autonomous Region, China -
Supported by:Guangxi Young Qihuang Scholars Training Program, No. GXQH202421 (to LZ); Guangxi Zhuang Autonomous Region Traditional Chinese Medicine Self-funded Scientific Research Project, No. GXZYK20230695 (to LZ); Guangxi Universities and Colleges Young and Middle-aged Teachers Scientific Research Basic Ability Enhancement Project, No. 2024KY0295 (to ZC); Guangxi Postgraduate Education Innovation Program Project, No. YCBXJ2023034 (to LZ); Doctoral Research Initiation Fund Project of Guangxi University of Chinese Medicine, No. 2023BS043 (to ZC); Guangxi Key Research Laboratory of Traditional Chinese Medicine for the Prevention and Treatment of Orthopedic and Traumatic Diseases (Cultivation), No. [2023]9 (to LZ)
CLC Number:
Cite this article
Liang Zhou, Zhang Chi, Pan Chengzhen, Yang Bo, Pu Zhanglin, Liu Hua, Peng Jinhui, Wen Lichun, Ling Guanhan, Chen Feng. Anti-osteoporotic mechanisms of kaempferol based on gut microbiota and comprehensive targeted metabolomics[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4190-4204.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
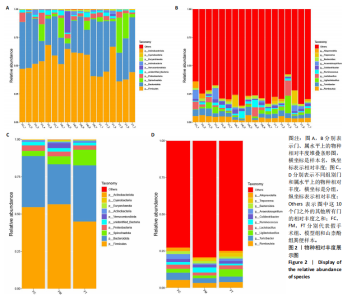
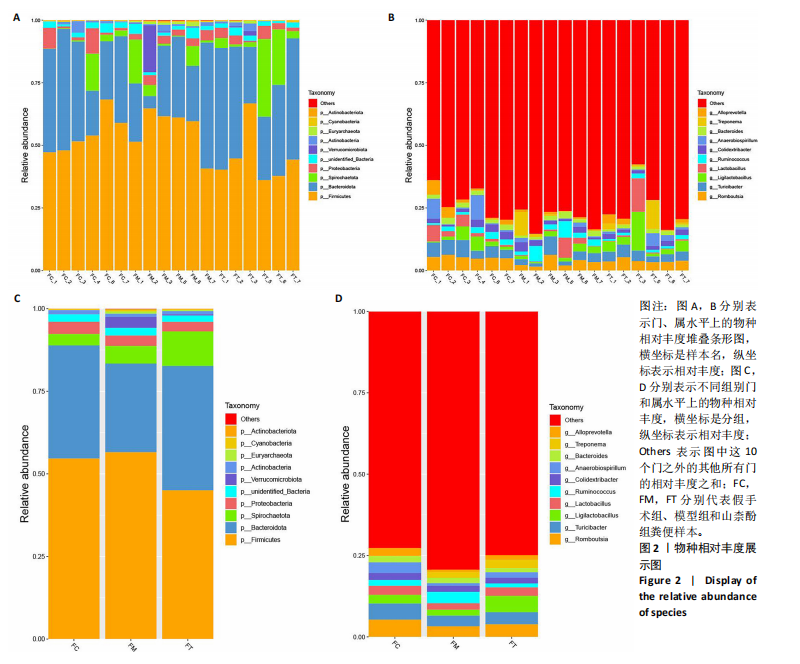
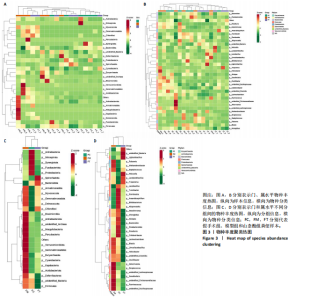
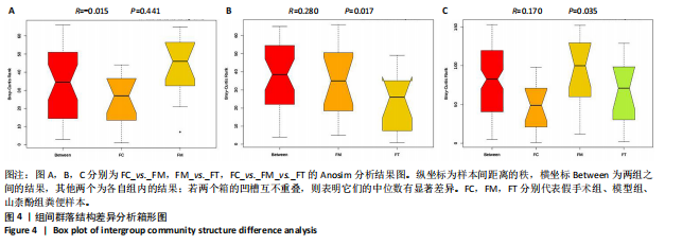
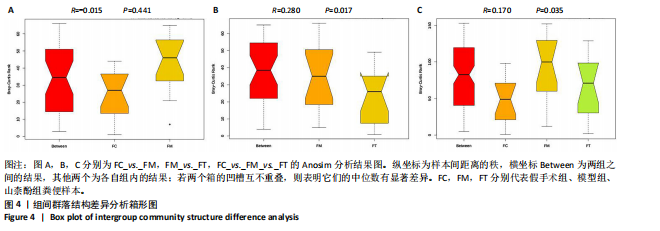
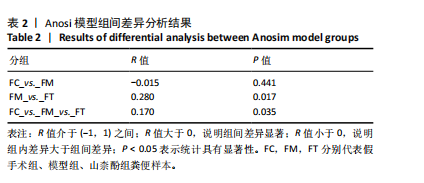
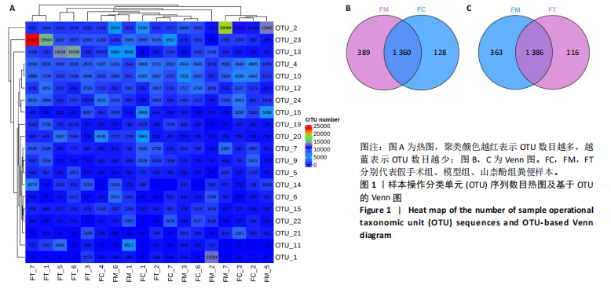
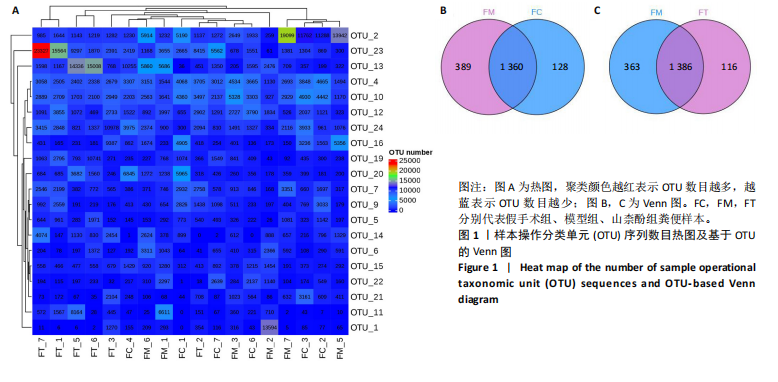
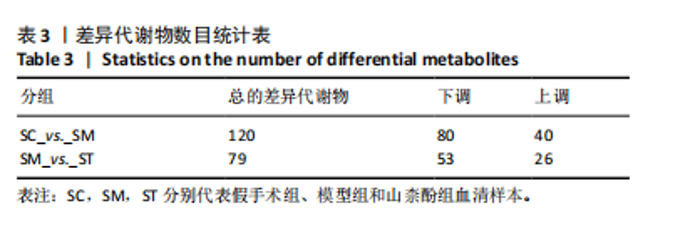
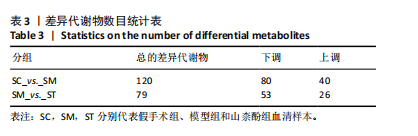
2.1 不同组别大鼠肠道菌群分析结果 2.1.1 菌群鉴定及物种分布 以97%的一致性为标准,对实验样本的Effective Tags实行OTUs聚类分析,分析每个样本的物种组成结构。对完成物种注释后的样本OTU数目进行统计,并绘制热图,见图1A。结果显示OUT23在山柰酚组的序列数目最多,OUT2在模型组的序列数目最多,两者皆包含拟杆菌门、拟杆菌纲、拟杆菌目和普雷沃氏菌科。OUT23在假手术组的序列数目最多,包括厚壁菌门、杆菌纲、链球菌目、链球菌科和土拉菌属。 根据聚类得到OTU结果,运用R3.5.0软件对模型组与假手术组、模型组和山柰酚组分别取交集,结果显示,模型组共有1 749个OUTs,假手术组共有1 488个OUTs;山柰酚组共有1 502个OUTs。其中模型组和假手术组具有1 360个交集OUTs,模型组和山柰酚组具有 1 386个交集OUTs,见图1B,C。 基于物种注释,从每个样本或分组中挑选出在各分类水平(门、纲、目、科、属、种)中丰度最高的前10种物种,并构建物种相对丰度累加柱状图,以直观展示不同分类水平上的主要物种及其相对比例。文章只展示门和属水平上各组肠道菌群的多样性和丰度。在门水平的分析中,丰度最高的前10种物种包括放线菌门(Actinobacteriota)、蓝细菌门(Cyanobacteria)、广古菌门(Euryarchaeota)、放线菌门(Actinobacteria)、疣微菌门(Verrucomicrobiota)、变形菌门(Proteobacteria)、螺旋菌门(spirochaetota)、拟杆菌门(Bacteroidota)和厚壁菌门(Firmicutes)。在属水平的分析中,丰度最高的前10种物种包括异普雷沃杆菌属(Alloprevotella)、梅毒螺旋体属(Treponema)、梭杆菌属(Fusobacterium)、厌氧螺旋菌属(Anaerobiospirillum)、结肠杆菌属(Eubacterium)、瘤胃球菌属(Ruminococcus)、乳杆菌属(Lactobacillus)、Lagilactobacillus属、土拉菌属(Turicibacter)及罗布特菌属(Romboutsia),见图2。 文章选取了所有样本中总定量值最高的35种微生物分类进行分析。利用这些微生物在各样本中的定量数据,从物种和样本两个维度进行聚类,并绘制热图。这种方法不仅便于识别哪些物种在特定样本中聚集或稀疏,还有助于评估样本间的聚类关系。结果显示与模型组相比,假手术组在门水平上放线菌(Actinobacteria)、副杆菌属(Fusobacterium)、疣状微生物群(Verrucomicrobiota)及酸细菌群(Acidobacteria)等丰度相对低表达,而在山柰酚组相对高表达。螺旋菌门(spirochaetota)、拟杆菌门(Bacteroidota)丰度在假手术组相对高表达,在山柰酚组相对低表达;属水平上阿克曼氏菌属(Akkermansia)、链球菌属(Streptococcus)、羊毛形杆菌科细菌属(Lachnospiraceae)、脱硫弧菌属(Desulfovibrio)及瘤胃球菌属(Ruminococcus)在假手术组相对高表达,在山柰酚组相对低表达。Latilactobacillus属、维瑟拉菌属(Veillonella)、植物乳杆菌属(Lactiplantibacillus)及梅毒螺旋体属(Treponema)丰度在假手术组相对低表达,在山柰酚组相对高表达。可以推断山柰酚可能通过调控上述菌群起到防治骨质疏松的作用,见图3。 2.1.2 组间群落结构差异显著性检验结果 采用Anosim分析来检验群落结构在组间的显著性差异。Anosim是一种非参数统计方法,其目的是判断组间差异是否显著高于组内差异,从而验证分组的有效性。该分析通过R3.5.0软件的vegan包实施,基于Bray-Curtis距离秩次进行显著性检验。分析结果根据样本间距离值的秩次排序,包括组间(between)和组内(within)差异,进而通过箱线图展示各组比较的统计数据。结果显示组间的差异显著大于组内差异,分组具备意义。且与假手术组相比,肠道菌群丰度在模型组上调,经山柰酚干预后,山柰酚组肠道菌群相较于模型组下调,见表2,图4。"
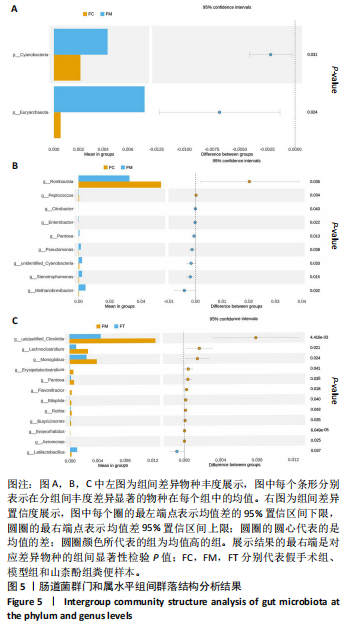
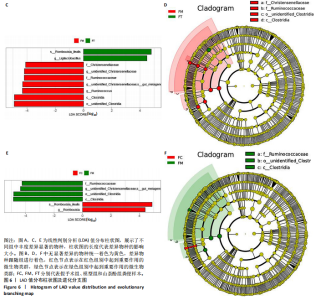
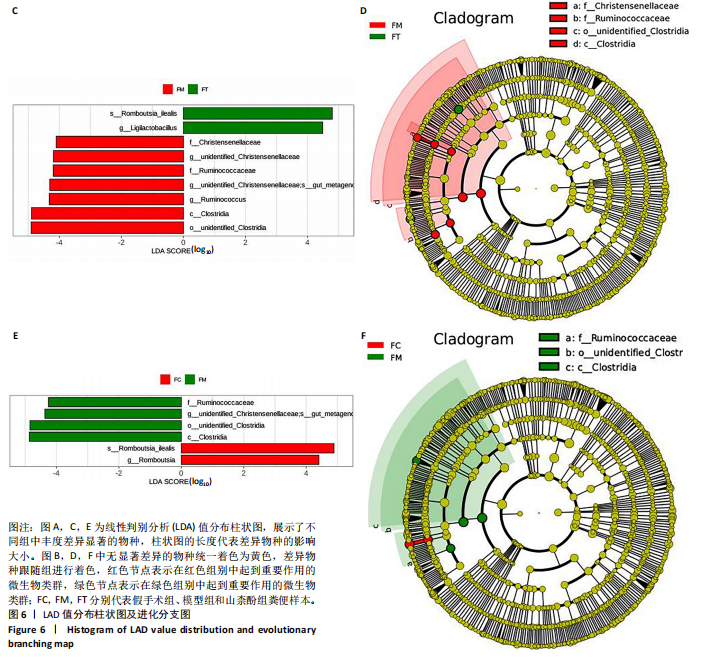
2.1.3 组间差异物种分析结果 在分组的项目中,通过群落结构差异的统计分析可以详细研究各组之间的差异。此类分析可有效识别各组间在物种丰度变化上显著不同的物种,并分析这些差异物种在不同组中的富集情况。同时,通过比较组内与组间的差异,可以评估不同分组间的群落结构是否存在统计学上的显著差异。 t检验分析结果:为了寻找在门和属分类水平上,组间的差异物种,故运用组间的t-test检验,找出差异显著(P < 0.05)的物种。差异分析结果表明模型组、假手术组、山柰酚组在门和属分类水平上的菌群差异有显著性意义。在门水平上,与假手术组相比,广古菌门(Euryarchaeota)(P=0.023)、蓝细菌门(Cyanobacteria)(P=0.030)在模型组上调,差异有显著性意义;在属水平上,与假手术组相比,模型组上调了甲烷小梭菌属(Methanobrevibacter)(P=0.031)、寡养单胞菌(Stenotrophomonas)(P=0.014)、蓝细菌门(Cyanobacteria)(P=0.030)、假单胞菌属(Pseudomonas)(P=0.038)、泛菌属(Pantoea)(P=0.012)、柠檬酸杆菌属(Citrobacter)(P=0.043)、肽球菌属(Peptococcus)(P=0.034)及肠杆菌属(Enterobacter)(P=0.021),下调了罗布特菌属(Romboutsia)(P=0.036)丰度,差异有显著性意义。模型组和山柰酚组相比,山柰酚上调Latilactobacillus属(P=0.021)丰度,下调泛菌属(Pantoea)(P=0.034)、肠杆菌属(Enterorhabdus)(P=0.000)、Monoglobu菌属(P=0.024)、丁酸单胞菌属(Butyricimonas)(P=0.034)、Rothia属(P=0.043)及梭杆菌属(Clostridia)(P=0.004)等菌属丰度,差异有显著性意义。其中肠杆菌属是3组之间共有的菌群,见图5。 LefSe分析结果:LEfSe是一种高维生物标志物(如基因、通路和分类单元)分析工具,用于比较两个或多个分组。该工具不仅注重统计意义,也强调生物学相关性,从而帮助研究人员识别组间在丰度上存在统计学差异的生物标记物。LEfSe的分析结果主要包括3个部分:LDA值的分布柱状图、系统发育分布的进化分支图,以及不"
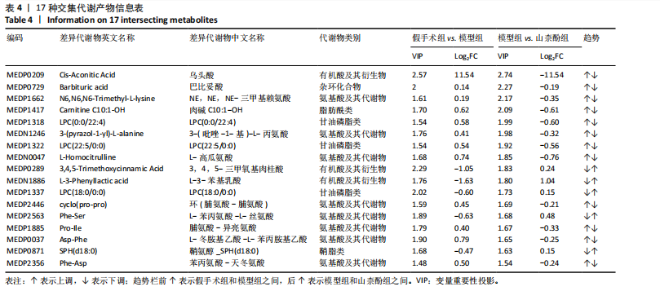
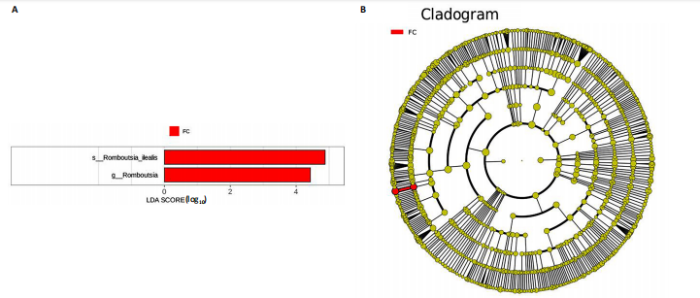
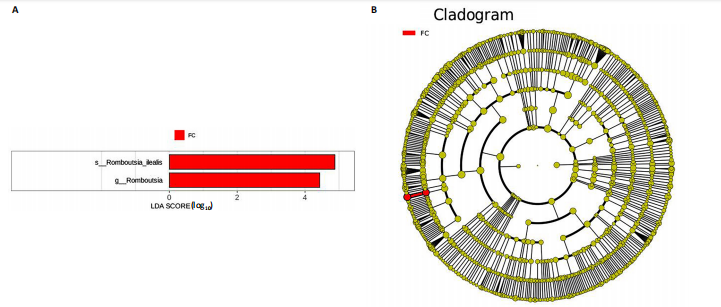
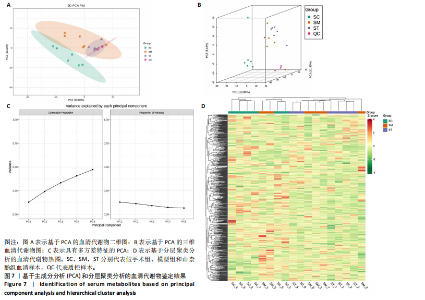
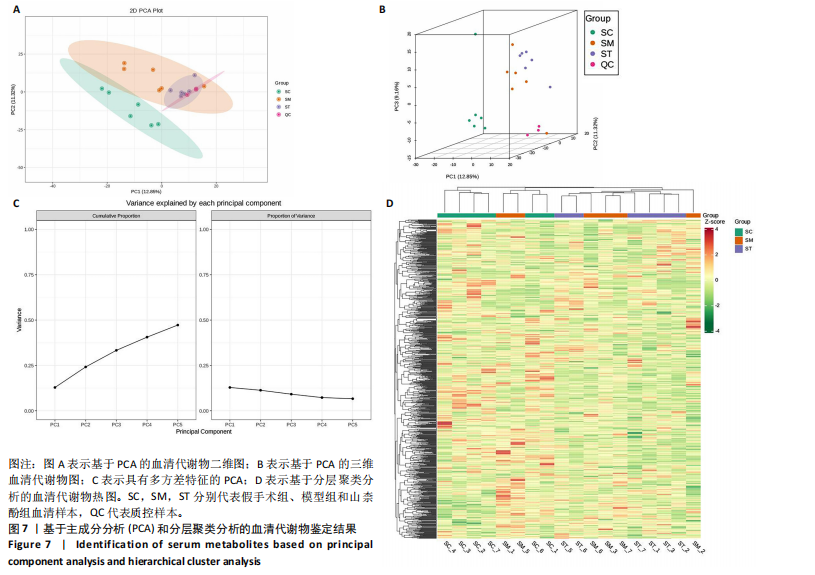
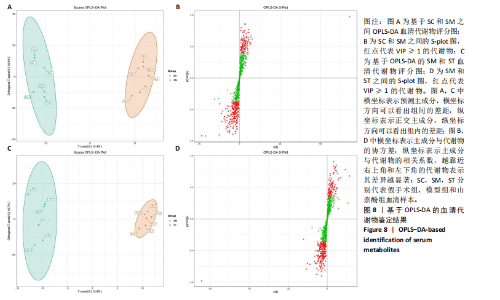
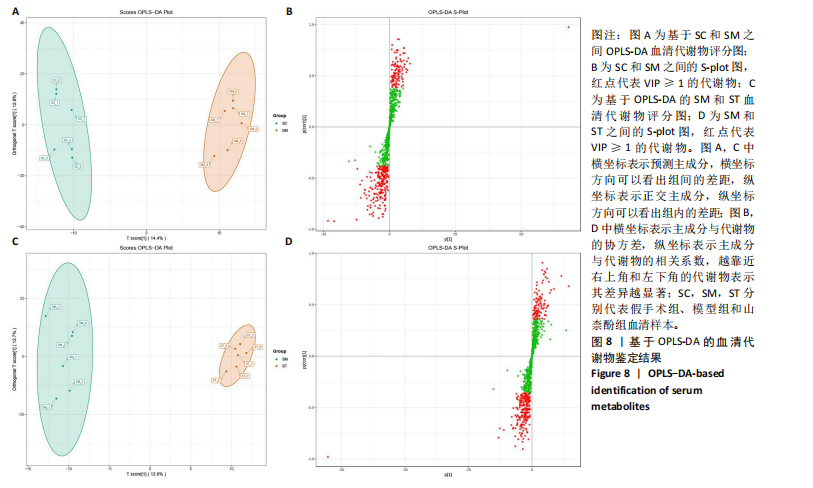
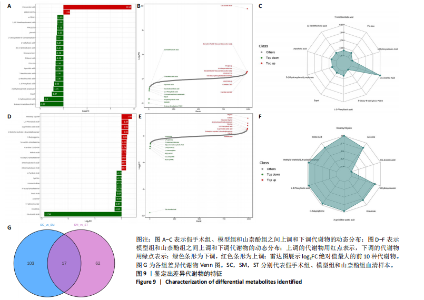
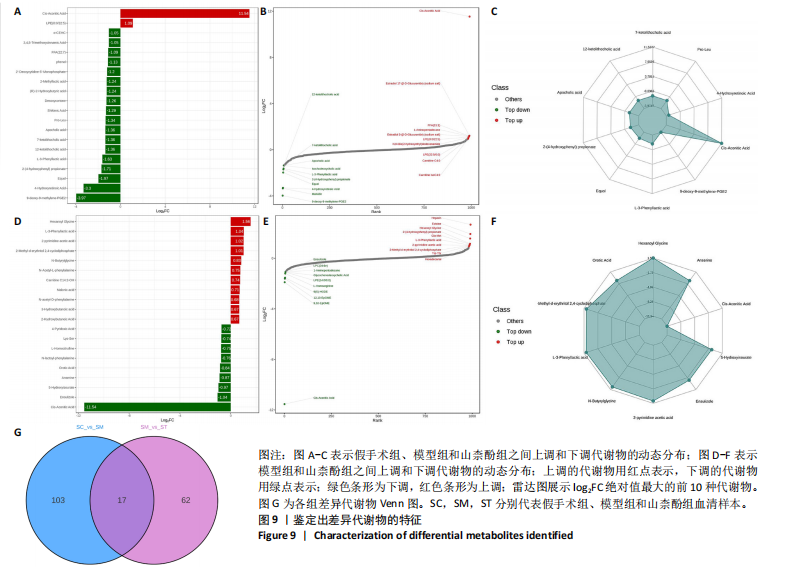
同组中具有显著性差异的生物标记物的丰度比较图。在文章中,当LDA得分超过4时,认为组间存在显著性差异。结果显示假手术组中起主要作用的菌群是罗布特菌属(Romboutsia),见图6A,B;通过模型组与山柰酚组对比,山柰酚组起主要作用的菌群是Lagilactobacillus菌属、罗布特菌属(Romboutsia)。模型组起主要作用的菌群是瘤胃球菌属(Ruminococcus)、梭菌纲(Clostridia)、克里斯滕森菌科(Christensenellaceae),见图6C,D。通过模型组与假手术组对比,在假手术组中起主要作用的菌群是罗布特菌属(Romboutsia),在模型组起主要作用的菌群是梭菌纲(Clostridia),见图6E,F。 2.2 不同组别大鼠血清样本广泛靶向代谢组学分析结果 实验在正负离子模式下共识别出991种化合物,其中正离子模式下识别了586种,而负离子模式下识别了405种。代谢物的组成具有样本特异性,不同类型的样本所包含的代谢物类别及比例不同。此外,在不同的处理或生物过程中,代谢物组成也会发生改变。通过代谢物组成比例分析,可以从整体上考察样本中的主要代谢物分布情况。结果显示PCA分析能明显区别假手术组和山柰酚组血清样本,无法清晰区分模型组和山柰酚组血清样本,见图7A-C。分层聚类的热图结果显示各组血清样本的代谢物之间具有表达差异,但无法清晰区别3组血清样本,见图7D。 鉴于PCA分析无法明显区分3组间的血清样本。故采用OPLS-DA加强模型组、假手术组及山柰酚组血清样本之间的差异。结果显示,通过OPLS-DA分析后的3组样本之间差异显著性增加,见图8A-D。假手术组和模型组的OPLS-DA得分分别为R2X=0.273,R2Y=0.99和Q2=0.607。模型组和山柰酚组之间OPLS-DA得分分别为R2X=0.254、R2Y=0.991和Q2=0.47。显示出较强的拟合度和预测能力。 假手术组与模型组相比、模型组与山柰酚组相比分别筛选出120和79种代谢物,见表3。假手术组和模型组之间的倍数变化值前10的上调代谢物分别是乌头酸、LPE(0:0/22:5);下调代谢物分别是(R)-2-羟基丁酸、12-酮基石胆酸、2'-脱氧胞苷-5'-单磷酸、2-(4-羟基苯基)丙酸酯、2-甲基乳酸、3,4,5-三甲氧基肉桂酸、4-羟视黄酸、7-酮基石胆酸、9-脱氧-9-亚甲基前列腺素E2、原胆酸、脱氧皮质酮、(S)-雌马酚、二十二碳七烯酸、L-3-苯基乳酸、脯氨酸-亮氨酸、莽草酸、苯酚及α-CEHC,见图9A-C。模型组和山柰酚组之间的倍数变化值前10的上调代谢物分别为2-羟基丁酸、2-甲基-d-赤藓糖醇2,4-环二磷酸、2-嘧啶乙酸、肉碱 C14:2-OH、己酰甘氨酸、L-3-苯基乳酸、丙二酸、正丁基甘氨酸及N-乙酰-D-苯丙氨酸。下调代谢物分别为3-羟基丁酸、4-吡哆酸、5-羟基异尿酸、鹅肌肽、乌头酸、2-苯基-5-苯并咪唑磺酸、L-高瓜氨酸、赖氨酸-丝氨酸、N-乙酰苯丙氨酸、N-乳酰基苯丙氨酸及乳清酸,见图9D-F。"
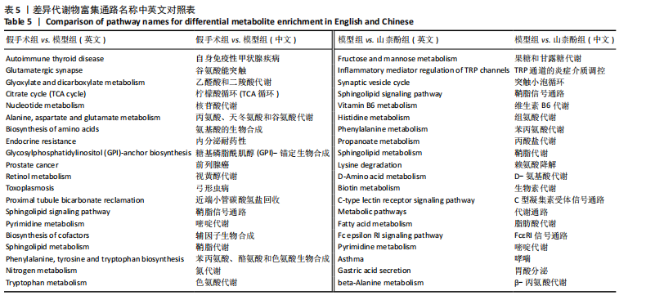
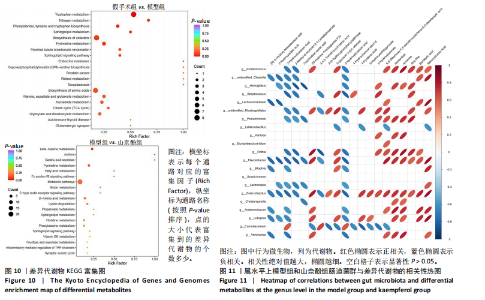
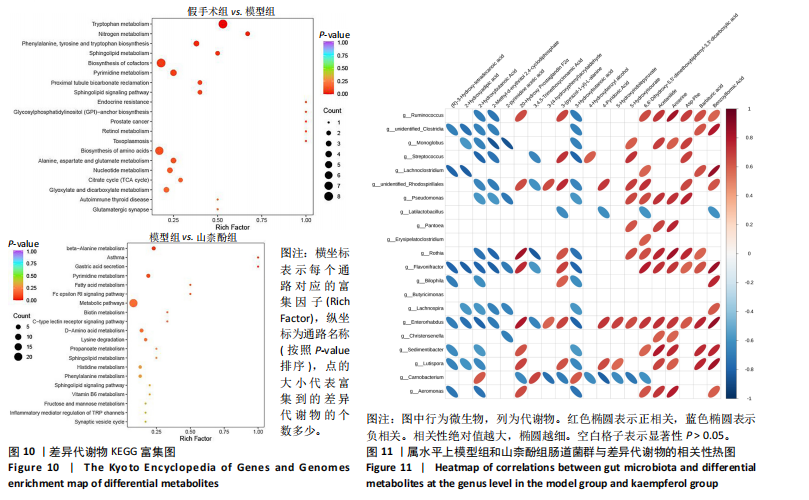
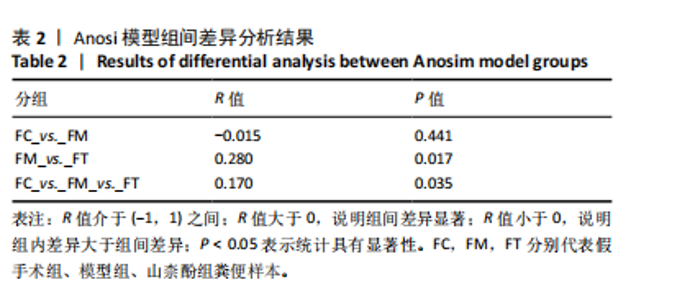
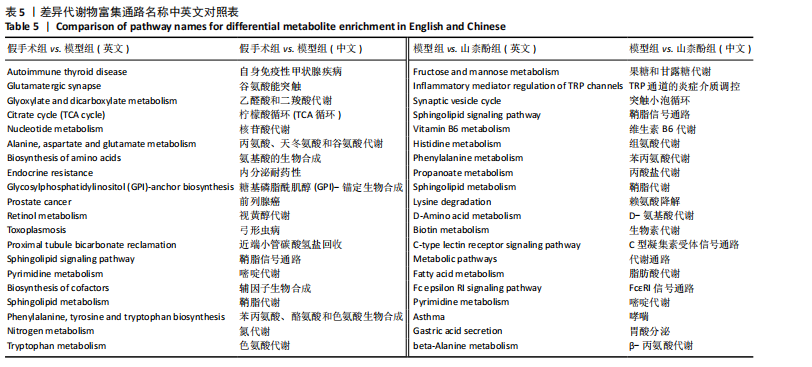
利用差异代谢物进行KEGG通路富集分析,发现假手术组和模型组相比,色氨酸代谢、鞘脂代谢、苯丙氨酸、酪氨酸和色氨酸生物合成、鞘脂信号通路丙氨酸、天冬氨酸和谷氨酸代谢及氨基酸的生物合成富集比较丰富,见图10A。模型组和山柰酚组相比,脂肪酸代谢、组氨酸代谢、鞘脂代谢、丙酸代谢、赖氨酸降解及D-氨基酸代谢富集比较丰富,见图10B。差异代谢物富集通路名称中英文对照见表5。 2.3 肠道菌群与代谢组学关联分析结果 山柰酚组在属水平调控的关键菌属为肠杆菌属(Enterorhabdus)、Latilactobacillus属、Rothia属、瘤胃球菌属(Ruminococcus)、链球菌属(Streptococcus)、假单胞菌属(Pseudomonas)、泛菌属(Pantoea)、丁酸单胞菌属(Butyricimonas)及克里斯滕森菌科(Christensenellaceae)等。其中肠杆菌属(Enterorhabdus)与4-吡哆酸(4-Pyridoxic Acid)、5-羟基吲哚丙酮酸(5-Hydroxyindolepyruvate)、5-羟基异尿酸(5-Hydroxyisourate)、6,6’-二羟基-5,5’-二甲氧基联苯-3,3’-二羧酸(6,6’-Dihydroxy-5,5’-dimethoxybiphenyl-3,3’-dicarboxylic acid)、乙酰苯胺(Acetanilide)、鹅肌肽(Anserine)、L-冬胺基乙酸-L-苯丙胺基乙酸(Aspartyl- Phenylalanine)、巴比妥酸(Barbituric acid)、苯乙醛酸(Benzoylformic Acid)正相关,而与(R)-3-羟基十四酸((R)-3-羟基十四酸)、2-羟基己二酸(2-Hydroxyadipic acid)、2-羟基丁酸(2-Hydroxybutanoic Acid)及2-甲基-D-赤藓糖醇2,4-环二磷酸((2-Methyl-d-erythritol 2,4-cyclodiphosphate)负相关,见图11。"
| [1] IOLOASCON G, DE SIRE A, CURCI C, et al. Osteoporosis guidelines from a rehabilitation perspective: systematic analysis and quality appraisal using AGREE II. Eur J Phys Rehabil Med. 2021;57(2):273-279. [2] STUMPF U, SCHMIDMAIER R. Sekundäre frakturprävention/update leitlinie osteoporose 2023 des dachverbandes osteologie [Secondary fracture prevention/update osteoporosis guidelines 2023 of the umbrella organization osteology]. Unfallchirurgie (Heidelb). 2024;127(4):283-289. [3] ZOU Z, LIU W, CAO L, et al. Advances in the occurrence and biotherapy of osteoporosis. Biochem Soc Trans. 2020;48(4):1623-1636. [4] REID IR. A broader strategy for osteoporosis interventions. Nat Rev Endocrinol. 2020;16(6):333-339. [5] PICKARD JM, ZENG MY, CARUSO R, et al. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279(1):70-89. [6] 钟建春,谢兴文,李鼎鹏,等.肠道菌群与骨质疏松的关系及中药调节研究进展[J].中国实验方剂学杂志,2022,28(18):237-244. [7] 韦露,马秀梅,覃树先,等.三种党参不同炮制品对脾虚模型大鼠肠道菌群的影响[J].湖北农业科学,2021,60(18):125-128. [8] CHEN Y, YANG C, DENG Z, et al. Gut microbially produced tryptophan metabolite melatonin ameliorates osteoporosis via modulating SCFA and TMAO metabolism. J Pineal Res. 2024;76(3):e12954. [9] HUSSAIN MS, ALTAMIMI ASA, AFZAL M, et al. Kaempferol: paving the path for advanced treatments in aging-related diseases. Exp Gerontol. 2024;188:112389. [10] GE J, LI G, CHEN Z, et al. Kaempferol and nicotiflorin ameliorated alcohol-induced liver injury in mice by miR-138-5p/SIRT1/FXR and gut microbiota. Heliyon. 2023;10(1):e23336. [11] GUAN M, XU W, BAI H, et al. Potential mechanisms underlying inhibition of xenograft lung cancer models by kaempferol: modulation of gut microbiota in activating immune cell function. J Cancer. 2024; 15(5):1314-1327. [12] PAN C, ZHANG C, LIN Z, et al. Disulfidptosis-related protein RPN1 may be a novel anti-osteoporosis targetof kaempferol. Comb Chem High Throughput Screen. 2024;27(11):1611-1628. [13] 唐雪勇,刁庆春,李鑫,等.基于广泛靶向代谢组学技术的寻常型银屑病血热证、血瘀证、血燥证患者唾液代谢组学研究[J].中医杂志,2021,62(9):789-795. [14] CAPORASO JG, LAUBER CL, WALTERS WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108 Suppl 1(Suppl 1):4516-4522. [15] CHEN Z, LV M, LIANG J, et al. Neuropeptide y-mediated gut microbiota alterations aggravate postmenopausal osteoporosis. Adv Sci (Weinh). 2023;10(33):e2303015. [16] 阙敏强,石础硕,黄英杰,等.不同药物治疗原发性骨质疏松症的网状Meta分析[J].中国组织工程研究,2020,24(35):5715-5722. [17] MCCLOSKEY E, TAN ATH, SCHINI M. Update on fracture risk assessment in osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2024;31(4): 141-148. [18] CHEN T, MENG F, WANG N, et al. The characteristics of gut microbiota and its relation with diet in postmenopausal osteoporosis. Calcif Tissue Int. 2024. doi: 10.1007/s00223-024-01260-x. [19] BATTISTINI C, BALLAN R, HERKENHOFF ME, et al. Vitamin d modulates intestinal microbiota in inflammatory bowel diseases. Int J Mol Sci. 2020;22(1):362. [20] MASSY ZA, DRUEKE TB. Gut microbiota orchestrates PTH action in bone: role of butyrate and T cells. Kidney Int. 2020;98(2):269-272. [21] ZHOU Y, ZHANG Y, QIAN Y, et al. Ziyuglycoside II attenuated OVX mice bone loss via inflammatory responses and regulation of gut microbiota and SCFAs. Int Immunopharmacol. 2024;132:112027. [22] CHANGF KY, LU CT, CHEN PC, et al. Correspondence: Association of estrogen deficiency on bone loss-impact of gut microbiota. Int J Rheum Dis. 2024;27(3):e15100. [23] SHIN SK, KWON EY. Kaempferol ameliorates metabolic syndrome by inhibiting inflammation and oxidative stress in high-fat diet-induced obese mice. Nutr Res Pract. 2024;18(3):325-344. [24] CHOI EY, HAN EJ, JEON SJ, et al. Kaempferol inhibits cervical cancer cells by inducing apoptosis and autophagy via inactivation of the PI3K/AKT/mTOR signaling pathway. Anticancer Res. 2024;44(7):2961-2972. [25] ALMATROUDI A, ALLEMAILEM KS, ALWANIAN WM, et al. Effects and mechanisms of kaempferol in the management of cancers through modulation of inflammation and signal transduction pathways. Int J Mol Sci. 2023;24(10):8630. [26] 杨启培,陈锋,崔伟,等.山奈酚活性单体治疗骨质疏松症的相关信号通路[J].中国组织工程研究,2024,28(26):4242-4249. [27] LI X, KHAN I, HUANG G, et al. Kaempferol acts on bile acid signaling and gut microbiota to attenuate the tumor burden in ApcMin/+ mice. Eur J Pharmacol. 2022;918:174773. [28] QU Y, LI X, XU F, et al. Kaempferol alleviates murine experimental colitis by restoring gut microbiota andinhibiting the LPS-TLR4-NF-κB axis. Front Immunol. 2021;12:679897. [29] SUN Y, ZHANG S, NIE Q, et al. Gut firmicutes: relationship with dietary fiber and role in host homeostasis. Crit Rev Food Sci Nutr. 2023;63(33): 12073-12088. [30] HOUTMAN TA, ECKERMANN HA, SMIDT H, et al. Gut microbiota and BMI throughout childhood: the role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci Rep.2022;12(1):3140. [31] TIAN H, CUI J, YE C, et al. Depletion of butyrate-producing microbes of the Firmicutes predicts nonresponse to FMT therapy in patients with recurrent Clostridium difficile infection. Gut Microbes. 2023;15(1): 2236362. [32] OSAWA M, HANDA O, FUKUSHIMA S, et al. Reduced abundance of butyric acid-producing bacteria in the ileal mucosa-associated microbiota of ulcerative colitis patients. J Clin Biochem Nutr. 2023; 73(1):77-83. [33] JI C, WU M, ZOU J, et al. Protection of γ-amino butyric acid on radiation induced intestinal injury in mice. Mol Nutr Food Res. 2023;67(10):e2200522. [34] LI Y, XING S, CHEN F, et al. Intracellular Fusobacterium nucleatum infection attenuates antitumor immunity inesophageal squamous cell carcinoma. Nat Commun. 2023;14(1):5788. [35] BOONYALEKA K, OKANO T, IIDA T, et al. Fusobacterium nucleatum infection activates the noncanonical inflammasome and exacerbates inflammatory response in DSS-induced colitis. Eur J Immunol. 2023; 53(11):e2350455. [36] CHUKKAPALLI SS, VELSKO IM, RIVERA-KWEH MF, et al. Global TLR2 and 4 deficiency in mice impacts bone resorption, inflammatory markers and atherosclerosis to polymicrobial infection. Mol Oral Microbiol. 2017;32(3):211-225. [37] ZHANG J, ZHANG Q, LIU H, et al. Soy-whey dual-protein alleviates osteoporosis of ovariectomized rats via regulating bone fat metabolism through gut-liver-bone axis. Nutrition. 2022;103-104:111723. [38] WANG S, WANG S, WANG X, et al. Effects of icariin on modulating gut microbiota and regulating metabolite alterations to prevent bone loss in ovariectomized rat model. Front Endocrinol (Lausanne). 2022;13:874849. [39] ZHANG J, ZHANG H, XIAO Y, et al. Interspecific differences and mechanisms of Lactobacillus-derived anti-inflammatory exopolysaccharides. Int J Biol Macromol. 2024;263(Pt 2):130313. [40] YAO M, LU Y, ZHANG T, et al. Improved functionality of Ligilactobacillus salivarius Li01 in alleviating colonic inflammation by layer-by-layer microencapsulation. NPJ Biofilms Microbiomes. 2021;7(1):58. [41] PIVA F, GERVOIS P, KARROUT Y, et al. Gut-joint axis: impact of bifidobacterial cell wall lipoproteins on arthritis development. Nutrients. 2023;15(23):4861. [42] FEICHAO S, RONGRONG C, SHICHANG J, et al. Analysis of the relationship between rheumatoid arthritis and osteoporosis based on mendelian randomization. Curr Rheumatol Rev. 2024;20(3):284-295. [43] KANITKAR A, CHEN C, SMOAK M, et al. In vitro characterization of polyesters of aconitic acid, glycerol, and cinnamic acid for bone tissue engineering. J Biomater Appl. 2015;29(8):1075-1078. [44] LIANG B, SHI X, WANG X, et al. Association between amino acids and recent osteoporotic fracture: a matched incident case-control study. Front Nutr. 2024;11:1360959. [45] LI SY, LU ZH, LEUNG JCS, et al. Association of dietary protein intake, inflammation with muscle mass, physical performance and incident sarcopenia in Chinese community-dwelling older adults. J Nutr Health Aging. 2024;28(4):100163. [46] CONIGRAVE AD, BROWN EM, RIZZOLI R. Dietary protein and bone health: roles of amino acid-sensing receptors in the control of calcium metabolism and bone homeostasis. Annu Rev Nutr. 2008;28:131-155. [47] GARTON M, NIM S, STONE TA, et al. Method to generate highly stable D-amino acid analogs of bioactive helical peptides using a mirror image of the entire PDB. Proc Natl Acad Sci U S A. 2018;115(7):1505-1510. [48] FITZPATRICK LA, BUZAS E, GAGNE TJ, et al. Targeted deletion of histidine decarboxylase gene in mice increases bone formation and protects against ovariectomy-induced bone loss. Proc Natl Acad Sci U S A. 2003;100(10):6027-6032. [49] XIE H, HUA Z, GUO M, et al. Gut microbiota and metabonomics used to explore the mechanism of Qing’e Pills in alleviating osteoporosis. Pharm Biol. 2022;60(1):785-800. [50] SU Y, ELSHORBAGY A, TURNER C, et al. Circulating amino acids are associated with bone mineral density decline and ten-year major osteoporotic fracture risk in older community-dwelling adults. Bone. 2019;129:115082. [51] DRAGOJEVIČ J, ZUPAN J, HARING G, et al. Triglyceride metabolism in bone tissue is associated with osteoblast and osteoclast differentiation: a gene expression study. J Bone Miner Metab. 2013;31(5):512-519. [52] SEAL A, HUGHES M, WEI F, et al. Sphingolipid-induced bone regulation and its emerging role in dysfunction due to disease and infection. Int J Mol Sci. 2024;25(5):3024. [53] LIN Z, JIANG T, CHEN M, et al. Gut microbiota and sleep: interaction mechanisms and therapeutic prospects. Open Life Sci. 2024;19(1): 20220910. [54] SEJBUK M, SIEBIESZUK A, WITKOWSKA AM. The role of gut microbiome in sleep quality and health: dietary strategies for microbiota support. Nutrients. 2024;16(14):2259. |
| [1] | Zhou Jinhai, Li Jiangwei, Wang Xuquan, Zhuang Ying, Zhao Ying, Yang Yuyong, Wang Jiajia, Yang Yang, Zhou Shilian. Three-dimensional finite element analysis of anterior femoral notching during total knee arthroplasty at different bone strengths [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1775-1782. |
| [2] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [3] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [4] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [5] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [6] | Li Yueyao, Zhang Min, Yang Jiaju. Cistanoside A mediates p38/MAPK pathway to inhibit osteoclast activity [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1144-1151. |
| [7] | Zheng Lin, Jin Wenjun, Luo Shanshan, Huang Rui, Wang Jie, Cheng Yuting, An Zheqing, Xiong Yue, Gong Zipeng, Liao Jian. Eucommia ulmoides promotes alveolar bone formation in ovariectomized rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1159-1167. |
| [8] |
Huang Xiaobin, Ge Jirong, Li Shengqiang, Xie Lihua, Huang Jingwen, He Yanyan, Xue Lipeng.
Mechanisms of different yin nourishing and kidney tonifying methods on osteoclastysis pathway in ovariectomized rats #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1214-1219.
|
| [9] | Qian Kun, Li Ziqing, Sun Shui . Endoplasmic reticulum stress in the occurrence and development of common degenerative bone diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1285-1295. |
| [10] |
Zhao Wensheng, Li Xiaolin, Peng Changhua, Deng Jia, Sheng Hao, Chen Hongwei, Zhang Chaoju, He Chuan.
Gut microbiota and osteoporotic fractures #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1296-1304.
|
| [11] | Lan Shuangli, Xiang Feifan, Deng Guanghui, Xiao Yukun, Yang Yunkang, Liang Jie. Naringin inhibits iron deposition and cell apoptosis in bone tissue of osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 888-898. |
| [12] |
Sun Guanghan, Xie Zhencong, Sun Mi, Xu Yang, Guo Dong.
Therapeutic effect and mechanism by which Trichosanthis Fructus-Allii Macrostemonis Bulbus regulates gut microbiota in a rat model of coronary heart disease #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 917-927.
|
| [13] | Wang Dongyang, Yang Qiaohui, Lin Xinchao. Relationship between vitamin D levels and reproductive characteristics and exercise dietary situation in postmenopausal women [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1021-1025. |
| [14] | Zhang Lichuang, Yang Wen, Ding Guangjiang, Li Peikun, Xiao Zhongyu, Chen Ying, Fang Xue, Zhang Teng. Dispersion effect of bone cement after vertebroplasty using individualized unilateral external pedicle approach and bilateral pedicle approach [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 800-808. |
| [15] | Zhang Xiongjinfu, Chen Yida, Cheng Xinyi, Liu Daihui, Shi Qin . Exosomes derived from bone marrow mesenchymal stem cells of young rats to reverse senescence in aged rat bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7709-7718. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||