Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (25): 5320-5327.doi: 10.12307/2025.530
Previous Articles Next Articles
M2 macrophage-derived exosomes promote microglia M2-type polarization
Fang Jun1, Wei Wei1, Xue Yating1, Cui Chenlong1, Wei Jiasheng1, Shi Xiao1, Yang Lijuan2, Yang Baozhong2
- 1Shanxi Medical University, Taiyuan 030032, Shanxi Province, China; 2Taiyuan Central Hospital, Taiyuan 030009, Shanxi Province, China
-
Received:2024-04-15Accepted:2024-06-11Online:2025-09-08Published:2024-12-19 -
Contact:Yang Baozhong, PhD, Chief physician, Taiyuan Central Hospital, Taiyuan 030009, Shanxi Province, China -
About author:Fang Jun, Master candidate, Shanxi Medical University, Taiyuan 030032, Shanxi Province, China -
Supported by:National Regional Medical Center Science and Technology Innovation Project, No. 202232, 202268 (to FJ)
CLC Number:
Cite this article
Fang Jun, Wei Wei, Xue Yating, Cui Chenlong, Wei Jiasheng, Shi Xiao, Yang Lijuan, Yang Baozhong. M2 macrophage-derived exosomes promote microglia M2-type polarization[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5320-5327.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
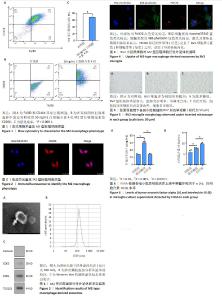
2.1 白细胞介素4诱导巨噬细胞M2型极化 通过流式细胞术对M2型巨噬细胞标志物进行检测,如图1A所示,用CD11b和F4/80标记骨髓原代巨噬细胞,圈出双阳性表达的细胞群即为巨噬细胞群,用CD206标记M2型巨噬细胞,结果如图1B所示,CD206阳性细胞群比例能达到68.4%。如图1C所示,与对照组相比,50 ng/mL白细胞介素4诱导24 h后M2型巨噬细胞比例显著升高(P < 0.000 1)。结果表明,白细胞介素4成功诱导骨髓原代巨噬细胞极化为M2型巨噬细胞。图2为细胞免疫荧光检测CD206阳性M2型巨噬细胞表达。 2.2 M2型巨噬细胞衍生外泌体鉴定结果 通过透射电镜观察M2型巨噬细胞衍生外泌体,如图3A所示,可观察到茶托状结构,符合典型的外泌体结构特点。通过纳米颗粒跟踪分析M2型巨噬细胞衍生外泌体,如图3B所示,粒径大小分布呈单峰状,平均粒径在140 nm左右,此粒径分析结果符合外泌体的大小属性。通过Western blot检测外泌体表面标志物蛋白CD63、CD81、TSG101,如图3C所示,外泌体表达CD63、CD81、TSG101,不表达Calnexin。结果表明,通过差速超速离心法成功提取到M2型巨噬细胞衍生外泌体。 2.3 BV2小胶质细胞对M2型巨噬细胞衍生外泌体的摄取实验 用激光共聚焦显微镜观察BV2细胞对于M2型巨噬细胞衍生外泌体的摄取情况,如图4所示,外泌体用PKH26红色荧光标记,BV2细胞核用Hoechst33342蓝色荧光标记,BV2细胞骨架用488-phalloidin绿色荧光标记,激光共聚焦显微镜扫描结果显示:PKH26标记的外泌体(红色)定位于小胶质细胞核周(蓝色)和细胞骨架(绿色)中间,证实了外泌体可以被BV2细胞摄取。 2.4 倒置显微镜观察BV2小胶质细胞形态改变 正常状态下,BV2细胞为半贴壁半悬浮细胞,悬浮细胞为圆形,贴壁细胞为圆形或梭形,与对照组相比,脂多糖组细胞胞体明显变粗,细胞突起变多变长,呈阿米巴样,而治疗组细胞形态相较脂多糖组显著改善,细胞多呈梭形,见图5。 2.5 ELISA检测细胞培养上清中炎症因子分泌水平 如图6A所示,与对照组相比,脂多糖组促炎因子肿瘤坏死因子α水平明显升高(P < 0.000 1),与脂多糖组相比,治疗组肿瘤坏死因子α水平明显降低(P < 0.000 1);如图6B所示,相较于脂多糖组,治疗组抗炎因子白细胞介素10水平明显升高(P < 0.001)。结果表明,M2巨噬细胞衍生外泌体可以降低促炎因子的表达而升高抗炎因子的表达。"

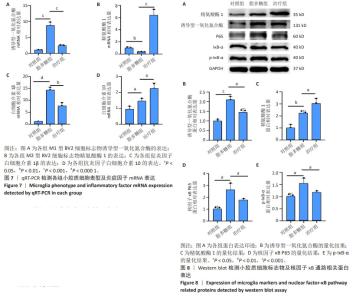
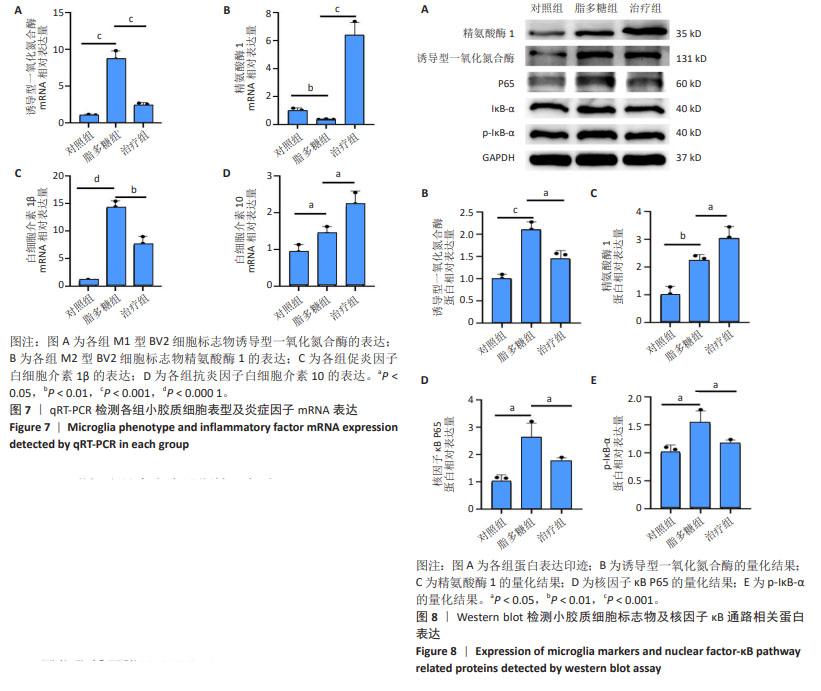
2.6 qRT-PCR检测小胶质细胞表型及炎症因子mRNA表达 如图7A所示,与对照组相比,脂多糖组M1型BV2细胞标志物诱导型一氧化氮合酶表达升高(P < 0.001),与脂多糖组相比,治疗组诱导型一氧化氮合酶表达降低(P < 0.001);如图7B所示,与脂多糖组相比,治疗组M2型BV2细胞标志物精氨酸酶1表达升高(P < 0.001);如图7C所示,与对照组相比,脂多糖组促炎因子白细胞介素1β表达明显升高(P < 0.000 1),与脂多糖组相比,治疗组白细胞介素1β表达明显降低(P < 0.01);如图7D所示,与脂多糖组相比,治疗组抗炎因子白细胞介素10表达升高(P < 0.05)。 2.7 Western blot检测小胶质细胞标志物及核因子κB通路相关蛋白表达 如图8A,B所示,与对照组相比,脂多糖组M1型小胶质细胞表面标志物诱导型一氧化氮合酶蛋白表达明显升高(P < 0.001),而经过M2巨噬细胞衍生外泌体处理之后,诱导型一氧化氮合酶蛋白表达明显降低(P < 0.05)。如图8C所示,与脂多糖组相比,治疗组M2型小胶质细胞表面标志物精氨酸酶1蛋白表达明显升高(P < 0.05)。对于核因子κB通路蛋白的表达,如图8D所示,与对照组相比,脂多糖组核因子κB P65蛋白表达明显升高(P < 0.05),而治疗组核因子κB P65蛋白表达明显降低(P < 0.05)。如图8E所示,与对照组相比,脂多糖组p-IκB-α蛋白表达明显升高(P < 0.05),而治疗组p-IκB-α蛋白表达明显降低(P < 0.05)。"
| [1] SUBHRAMANYAM CS, WANG C, HU Q, et al. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol. 2019;94:112-120. [2] TSUDA M, MASUDA T, KOHNO K. Microglial diversity in neuropathic pain. Trends Neurosci. 2023;46(7):597-610. [3] 方茜,吴文,唐丹喆.脊髓小胶质细胞的极化对神经病理性疼痛的影响[J].中国疼痛医学杂志,2022,28(4):285-289. [4] HU X, DU L, LIU S, et al. A TRPV4-dependent neuroimmune axis in the spinal cord promotes neuropathic pain. J Clin Invest. 2023;133(5): e161507. [5] ZHANG LQ, GAO SJ, SUN J, et al. DKK3 ameliorates neuropathic pain via inhibiting ASK-1/JNK/p-38-mediated microglia polarization and neuroinflammation. J Neuroinflammation. 2022;19(1):129. [6] LONG Y, LI XQ, DENG J, et al. Modulating the polarization phenotype of microglia - A valuable strategy for central nervous system diseases. Ageing Res Rev. 2024;93:102160. [7] LAN X, HAN X, LI Q, et al. Pinocembrin protects hemorrhagic brain primarily by inhibiting toll-like receptor 4 and reducing M1 phenotype microglia. Brain Behav Immun. 2017;61:326-339. [8] SI W, LI X, JING B, et al. Stigmasterol regulates microglial M1/M2 polarization via the TLR4/NF-κB pathway to alleviate neuropathic pain. Phytother Res. 2024;38(1):265-279. [9] MISHRA A, BHARTI PS, RANI N, et al. A tale of exosomes and their implication in cancer. Biochim Biophys Acta Rev Cancer. 2023;1878(4): 188908. [10] KRYLOVA SV, FENG D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int J Mol Sci. 2023;24(2):1337. [11] ISAAC R, REIS FCG, YING W, et al. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33(9):1744-1762. [12] JEPPESEN DK, FENIX AM, FRANKLIN JL, et al. Reassessment of Exosome Composition. Cell. 2019;177(2):428-445.e18. [13] CUI Y, HONG S, XIA Y, et al. Melatonin Engineering M2 Macrophage-Derived Exosomes Mediate Endoplasmic Reticulum Stress and Immune Reprogramming for Periodontitis Therapy. Adv Sci (Weinh). 2023;10(27):e2302029. [14] GENTEK R, MOLAWI K, SIEWEKE MH. Tissue macrophage identity and self-renewal. Immunol Rev. 2014;262(1):56-73. [15] ALLES SRA, SMITH PA. Etiology and Pharmacology of Neuropathic Pain. Pharmacol Rev. 2018;70(2):315-347. [16] DINI L, TACCONI S, CARATA E, et al. Microvesicles and exosomes in metabolic diseases and inflammation. Cytokine Growth Factor Rev. 2020;51:27-39. [17] FU SP, CHEN SY, PANG QM, et al. Advances in the research of the role of macrophage/microglia polarization-mediated inflammatory response in spinal cord injury. Front Immunol. 2022;13:1014013. [18] SHAPOURI-MOGHADDAM A, MOHAMMADIAN S, VAZINI H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425-6440. [19] ZHAO W, MA L, DENG D, et al. M2 macrophage polarization: a potential target in pain relief. Front Immunol. 2023;14:1243149. [20] SUH JH, JOO HS, HONG EB, et al. Therapeutic Application of Exosomes in Inflammatory Diseases. Int J Mol Sci. 2021;22(3):1144. [21] ZHAO R, ZHAO T, HE Z, et al. Composition, isolation, identification and function of adipose tissue-derived exosomes. Adipocyte. 2021; 10(1):587-604. [22] HU X, LEAK RK, SHI Y, et al. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015;11(1):56-64. [23] ATTA AA, IBRAHIM WW, MOHAMED AF, et al. Microglia polarization in nociplastic pain: mechanisms and perspectives. Inflammopharmacology. 2023;31(3):1053-1067. [24] JURGA AM, PALECZNA M, KUTER KZ. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front Cell Neurosci. 2020;14:198. [25] INOUE K, TSUDA M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 2018;19(3):138-152. [26] KOBAYASHI M, KONISHI H, SAYO A, et al. TREM2/DAP12 Signal Elicits Proinflammatory Response in Microglia and Exacerbates Neuropathic Pain. J Neurosci. 2016;36(43):11138-11150. [27] XU F, HUANG J, HE Z, et al. Microglial polarization dynamics in dorsal spinal cord in the early stages following chronic sciatic nerve damage. Neurosci Lett. 2016;617:6-13. [28] LI X, SHI H, ZHANG D, et al. Paeonol alleviates neuropathic pain by modulating microglial M1 and M2 polarization via the RhoA/p38MAPK signaling pathway. CNS Neurosci Ther. 2023;29(9):2666-2679. [29] WU Q, ZHENG Y, YU J, et al. Electroacupuncture alleviates neuropathic pain caused by SNL by promoting M2 microglia polarization through PD-L1. Int Immunopharmacol. 2023;123:110764. [30] GIRIDHARAN S, SRINIVASAN M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J Inflamm Res. 2018;11: 407-419. [31] YANG H, WU L, DENG H, et al. Anti-inflammatory protein TSG-6 secreted by bone marrow mesenchymal stem cells attenuates neuropathic pain by inhibiting the TLR2/MyD88/NF-κB signaling pathway in spinal microglia. J Neuroinflammation. 2020;17(1):154. [32] ZHENG Y, ZHAO J, CHANG S, et al. β-Sitosterol Alleviates Neuropathic Pain by Affect Microglia Polarization through Inhibiting TLR4/NF-κB Signaling Pathway. J Neuroimmune Pharmacol. 2023;18(4):690-703. [33] MOKHTARI T, YUE LP, HU L. Exogenous melatonin alleviates neuropathic pain-induced affective disorders by suppressing NF-κB/ NLRP3 pathway and apoptosis. Sci Rep. 2023;13(1):2111. [34] MORINAGA H, MAYORAL R, HEINRICHSDORFF J, et al. Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes. 2015;64(4):1120-1130. [35] YING W, GAO H, DOS REIS FCG, et al. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab. 2021;33(4):781-790.e5. [36] PAJARINEN J, LIN T, GIBON E, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80-89. [37] WANG Y, LIN Q, ZHANG H, et al. M2 macrophage-derived exosomes promote diabetic fracture healing by acting as an immunomodulator. Bioact Mater. 2023;28:273-283. [38] CHEN X, WAN Z, YANG L, et al. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J Nanobiotechnology. 2022;20(1):110. [39] LIU Y, LI YP, XIAO LM, et al. Extracellular vesicles derived from M2 microglia reduce ischemic brain injury through microRNA-135a-5p/TXNIP/NLRP3 axis. Lab Invest. 2021;101(7):837-850. [40] SONG Y, LI Z, HE T, et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics. 2019;9(10):2910-2923. |
| [1] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [2] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| [3] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [4] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [5] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [6] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [7] | He Longcai, Song Wenxue, Ming Jiang, Chen Guangtang, Wang Junhao, Liao Yidong, Cui Junshuan, Xu Kaya. An experimental method for simultaneous extraction and culture of primary cortical neurons and microglial cells from SD rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1395-1400. |
| [8] | Chang Jinxia, Liu Yufei, Niu Shaohui, Wang Chang, Cao Jianchun. Visualization analysis of macrophage polarization in tissue repair process [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1486-1496. |
| [9] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| [10] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [11] | Ji Huihui, Jiang Xu, Zhang Zhimin, Xing Yunhong, Wang Liangliang, Li Na, Song Yuting, Luo Xuguang, Cui Huilin, Cao Ximei. SR9009 combined with indolepropionic acid alleviates inflammation in C2C12 myoblasts through the nuclear factor-kappa B signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1220-1229. |
| [12] | Bai Jing, Zhang Xue, Ren Yan, Li Yuehui, Tian Xiaoyu. Effect of lncRNA-TNFRSF13C on hypoxia-inducible factor 1alpha in periodontal cells by modulation of #br# miR-1246 #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 928-935. |
| [13] | Zhi Fang, Zhu Manhua, Xiong Wei, Lin Xingzhen. Analgesic effect of acupuncture in a rat model of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 936-941. |
| [14] | Wang Yuru, Li Siyuan, Xu Ye, Zhang Yumeng, Liu Yang, Hao Huiqin. Effects of wogonin on joint inflammation in collagen-induced arthritis rats via the endoplasmic reticulum stress pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1026-1035. |
| [15] | Wang Sifan, He Huiyu, Yang Quan, Han Xiangzhen. miRNA-378a overexpression of macrophage cell line composite collagen sponge: anti-inflammation and tissue repair promotion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 789-799. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||