Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (21): 4576-4583.doi: 10.12307/2025.809
Previous Articles Next Articles
Role of neuropilin 1 in promoting angiogenesis-osteogenesis coupling during fracture healing
Zheng Li1, Ding Yiheng2, Li Xinhao2, Wen Zekai2, Jiang Bingzheng1, Lin Xuexia1
- 1Xingtai Medical College, Xingtai 054000, Hebei Province, China; 2Hebei Key Lab of Laboratory Animal Science, Department of Experimental Animal Science of Hebei Medical University, Shijiazhuang 050017, Hebei Province, China
-
Received:2024-05-23Accepted:2024-07-10Online:2025-07-28Published:2024-12-07 -
Contact:Jiang Bingzheng, MS, Professor, Xingtai Medical College, Xingtai 054000, Hebei Province, China Lin Xuexia, MS, Professor, Xingtai Medical College, Xingtai 054000, Hebei Province, China -
About author:Zheng Li, MS, Associate professor, Xingtai Medical College, Xingtai 054000, Hebei Province, China -
Supported by:Key Research and Development Program of Hebei Province, No. 223777133D (to ZL)
CLC Number:
Cite this article
Zheng Li, Ding Yiheng, Li Xinhao, Wen Zekai, Jiang Bingzheng, Lin Xuexia. Role of neuropilin 1 in promoting angiogenesis-osteogenesis coupling during fracture healing[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(21): 4576-4583.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
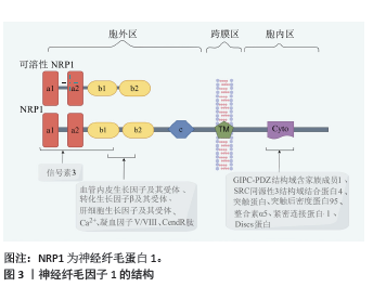
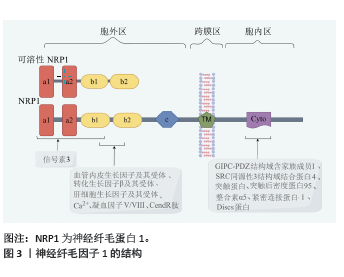
2.1 神经纤毛因子1结构 神经纤毛因子1最初是作为神经元导向和突触形成的因子而被人们认识,近年来人们发现神经纤毛因子1在骨折愈合过程中也发挥重要的作用。 神经纤毛因子1存在于大部分的脊椎动物中,是相对分子质量约为1.3×105的跨膜糖蛋白,神经纤毛因子1分为胞外区、跨膜区和胞内区3部分。神经纤毛因子1蛋白从N端到C端分别是a1、a2、b1、b2、c、跨膜区、胞内区。a1和a2主要结合信号素3,少部分信号素3也结合b1,b1 和 b2 主要结合凝血因子Ⅴ/Ⅷ、血管内皮生长因子及其受体、转化生长因子β及其受体、血小板衍生生长因子及其受体、肝细胞生长因子及其受体、Ca2+、CendR肽等符合C端规则的因子[2-4];疏水的跨膜区参与神经纤毛因子1二聚体化。胞内区包含一个有突触后密度蛋白95、Discs蛋白和紧密连接蛋白ZO-1组成的PDZ膜序,羧基端有丝氨酸谷氨酸丙氨酸三联体[2]。胞内区可结合多因子而发挥作用,例如GIPC-PDZ结构域含家族成员1、SRC同源性3结构域结合蛋白4、突触蛋白、整合素α5、突触后密度蛋白95、Discs蛋白和紧密连接蛋白ZO-1等[5-7]。另外,神经纤毛因子1还结合Raft交联蛋白和半乳糖凝集素1 [8-9]。见图3(此图由Figdraw绘制)。神经纤毛因子1蛋白一旦合成很稳定,主要通过Ras相关蛋白Rab11A依赖性途径(“慢循环”)进行蛋白回收[10]。神经纤毛因子1蛋白翻译后进行包括糖胺聚糖修饰等糖基化修饰及甲基化修饰等[11-12]。"
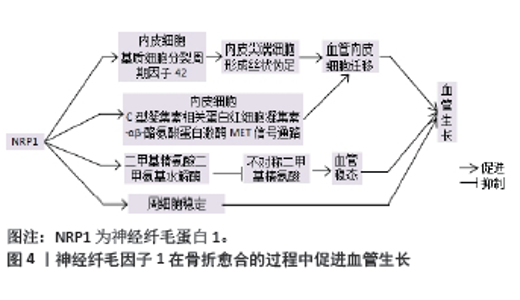
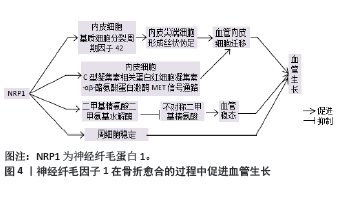
2.2 神经纤毛因子1在骨折愈合中促进血管再生 2.2.1 神经纤毛因子1与动脉毛细血管细胞 毛细动脉血管生成是骨折愈合的关键事件,毛细动脉血管由内皮细胞和周细胞组成。神经纤毛因子1缺陷会导致外周微血管病变和血管生成障碍[13-14]。神经纤毛因子1促进内皮细胞迁移,在细胞迁移过程中,神经纤毛因子1激活细胞外基质中的细胞分裂周期因子42,使内皮尖端细胞形成丝状伪足,从而促进血管内皮迁移[15]。REZAEI等[4]研究发现在人脐静脉血管内皮细胞中,C型凝集素相关蛋白视多西素-αβ诱导神经纤毛因子1激活下游通路,导致细胞骨架增多、细胞硬度增强,促进了细胞间力传递,加速血管内皮细胞的迁移。神经纤毛因子1上调二甲基精氨酸二甲氨基水解酶的表达,降解不对称二甲基精氨酸蛋白,从而发挥维持血管稳态的作用 [16]。见图4。"

周细胞是包裹毛细血管和小静脉内皮细胞的可收缩细胞,在维持血管稳定性和完整性方面发挥着至关重要的作用。神经纤毛因子1对周细胞的稳定有重要作用。经己烯雌酚治疗的小鼠,出现子宫毛细血管幼稚化以及周细胞较少的现象;这是因为血管内皮因子受体2和共受体神经纤毛因子1等血管生成因子浓度低,最终造成血管密度低和脉管系统不成熟[17]。 2.2.2 神经纤毛因子1与两种生长因子 在骨折愈合的过程中,神经纤毛因子 1 通过促进血管内皮细胞和周细胞生长而发挥促血管作用,这种作用与血管内皮生长因子和血小板衍生生长因子这两种因子密切相关。首先,神经纤毛因子1通过血管内皮生长因子、血小板衍生生长因子途径发挥促进血管内皮生长的作用。血管内皮生长因子包括A-E以及胎盘生长因子共6种,神经纤毛因子1可以通过血管内皮生长因子促进血管出芽、成管[18]。血管内皮生长因子受体有3种,其中血管内皮生长因子受体1,2与血管内皮细胞关系紧密,而血管内皮生长因子受体3与淋巴内皮细胞关联较多。在没有内皮糖蛋白等配体情况下,神经纤毛因子1优先与细胞内的血管内皮生长因子受体1结合;在有配体情况下,神经纤毛因子1优先与细胞膜上的血管内皮生长因子受体2结合[10,18]。神经纤毛因子1与血管内皮生长因子及其受体等形成复合物会进一步募集相关因子,例如神经纤毛因子1/血管内皮生长因子复合物触发胰岛素样生长因子1受体相互作用蛋白1、突触蛋白、Ras同源基因家族成员A/Ras同源基因家族成员A相关蛋白激酶、有丝分裂原活化蛋白激酶激酶3/6级联反应来激活p38丝裂原活化蛋白激酶[7]。COLOTTI等[19]发现神经纤毛因子1与血管内皮生长因子受体1,2互相作用,并且参与整合素α5β1/可溶性血管内皮生长因子受体1复合物介导的内皮细胞黏附和迁移。在血管的生成过程中,神经纤毛因子1与血管内皮生长因子关系密切,共同发挥促进血管生长的作用。H型血管作为毛细血管的一种亚型,可能也接受神经纤毛因子1与血管内皮生长因子的促进作用。血小板衍生生长因子可促进内皮祖细胞的迁移和分化,增强了H型血管和骨骼的形成[20]。血小板衍生生长因子BB可诱导骨膜蛋白表达和募集,促进骨和H型血管再生[21]。神经纤毛因子1可通过血小板衍生生长因子发挥促进H型血管生长的作用。 其次,血小板衍生生长因子与周细胞之间有密切的关系。神经纤毛因子1与血小板衍生生长因子D结合可移位到内皮细胞的连接处。并且,在血小板衍生生长因子D的诱导下,神经纤毛因子1与血小板衍生生长因子受体β相互作用(在内皮细胞和周细胞之间),作为血小板衍生生长因子D-血小板衍生生长因子受体β的辅助受体,在周细胞的细胞通讯中发挥一定作用[22]。DHAR等[23]研究发现神经纤毛因子1与血小板衍生生长因子B互相作用,将周细胞募集至新生血管处,促进血管再生。 2.2.3 神经纤毛因子1与H型血管 H型血管由垂直排列的血管柱组成,并且通过血管环或拱门连接形成网状结构,是一类高表达血小板内皮细胞黏附分子和内皮黏蛋白的动脉毛细血管,主要由血管内皮细胞和周细胞组成,主要存在于长骨的干骺端和内膜等骨生成活跃的区域,在骨-血管偶联中起到关键作用,也是促进骨折愈合的新靶点之一[24-30]。H型血管是动脉毛细血管的一种亚型,神经纤毛因子1促进动脉毛细血管中内皮细胞迁移和周细胞稳定性,而神经纤毛因子1是否促进H型血管生成有待进一步研究。破骨细胞、成骨细胞、软骨细胞和内皮细胞通过分泌血小板衍生生长因子BB促进H型血管生长,内皮细胞还可以分泌血管内皮生长因子促进H型血管的生长,而神经纤毛因子1作为2种因子的共受体,是否也通过血管内皮生长因子途径、血小板衍生生长因子途径促进H型血管生长有待进一步研究。 2.3 神经纤毛因子1在骨折愈合中对骨的作用 2.3.1 神经纤毛因子1与骨细胞 成骨细胞、破骨细胞、肥大软骨细胞等是完成骨折愈合的主要骨细胞,其中成骨细胞是数量最多的细胞。成骨细胞和胶原纤维组成编织骨,形成板状骨,最终完成骨折愈合。破骨细胞不仅吸收骨折端无活动的骨片,而且会在骨折断端堆积Ca2+,为成骨细胞提供必需的营养。肥大软骨细胞在软骨痂变为硬骨痂时候逐渐凋亡,协同编织骨的形成。 在骨折愈合过程中,骨细胞中神经纤毛因子1有着重要的作用。CHOE [31]研究发现内胚层缺乏神经纤毛因子1会导致斑马鱼面部软骨严重缺陷。ZHOU等[32]以中国汉族400名绝经后妇女为研究对象,采用靶向二代测序技术进行检测,发现神经纤毛因子1 rs2070296与股骨颈的骨密度/骨质疏松风险显著关联、神经纤毛因子1 rs180868035与腰椎和股骨颈的骨密度显著关联;神经纤毛因子1 rs180868035转染的成骨细胞/破骨细胞与野生型相比,突变细胞的成骨标志物表达降低而破骨标志物的表达升高;另外,突变体神经纤毛因子1 rs180868035转染减缓成骨细胞增殖,促进其凋亡,而对破骨细胞有相反作用。HAYASHI等[33]通观察肢体间充质中信号素3A表达缺失的转基因小鼠出现骨体积显著降低、骨吸收高、骨形成低的情况,进而观察信号素 3A与神经纤毛因子1作用被切断的转基因小鼠,发现小鼠的骨量减少;他们采用可溶性鸟苷酸环化酶信号刺激因子模拟信号素3A的作用,结果显示神经纤毛因子1表达升高,骨质疏松症得到改善。ZHANG等[34]发现MC3T3成骨细胞在高糖干预下,信号素3A及神经纤毛因子1的表达降低,成骨分化被抑制;通过补充神经纤毛因子1的配体SEMA3A,可增加MC3T3以及糖尿病动物中的成骨细胞标志物表达,同时动物的骨密度和骨量也增加。PAN等[35] 研究发现敲除miR-148a的小鼠破骨细胞减少、骨量增加;而神经纤毛因子1是miR-148a的靶点蛋白,即敲除 miR-148a会诱导神经纤毛因子1表达升高从而增加骨量。木犀草素通过促进信号素3A、神经纤毛因子1表达最终增强了成骨细胞标志物的表达[36]。神经纤毛因子1过表达会引起成骨细胞中碱性磷酸酶、骨钙素、骨保护素、Runt相关转录因子2、骨形态发生蛋白2等成骨标志蛋白表达上调和破骨细胞的形成和分化减缓,从而最终起到增加骨量的作用[36-37]。神经纤毛因子1高表达会减少脂多糖对ATDC5软骨细胞样细胞的损伤,减少细胞凋亡和细胞外基质降解[38]。神经纤毛因子1低表达导致成骨细胞数量减少,软骨细胞增殖率降低,细胞黏附增加,细胞衰老程度增加[35,39]。髓核祖细胞/间充质干细胞通过神经纤毛因子1相关的级联反应发挥骨修复和维持骨稳态作用[40]。另外,H型血管的内皮细胞表达更高的转化生长因子β,这些因子可以促进骨祖细胞存活和增殖[24],而神经纤毛因子1也是转化生长因子β的受体,神经纤毛因子1是否通过H型血管促进骨细胞生长有待进一步研究。具体见表1。"


2.3.2 在骨折愈合过程中发挥作用的神经纤毛因子1的上下游分子 在骨折愈合过程中,神经纤毛因子1上下游有一些关键分子。神经纤毛因子1上游正调控因子有肺腺癌转移性相关转录本1等 [41],神经纤毛因子1负调控因子有miR-148a、miR-320a、miR-485-3p等[35,41-42]。神经纤毛因子1下游有神经丛蛋白A1、盘状蛋白结构域受体酪氨酸激酶2以及Runt相关转录因子2、骨钙素、骨桥蛋白等骨标志蛋白。神经纤毛因子1通过抑制盘状蛋白结构域受体酪氨酸激酶2的降解,促进其表达[43]。神经纤毛因子1正调控Runt相关转录因子2、骨钙素、骨桥蛋白、细胞外信号调节激酶[43-44]。具体见表2。 2.4 神经纤毛因子1在骨折愈合中促进骨-血管偶联 2.4.1 神经纤毛因子1是成骨-破骨细胞偶联的关键因子 神经纤毛因子1作为信号素3A的受体,既可以通过抑制破骨细胞的核因子κB受体活化因子配体抑制破骨细胞分化,也可以通过WNT/β-连环蛋白等途径促进成骨分化[44]。神经纤毛因子1是调节性T细胞的表面标志物。免疫细胞的神经纤毛因子1与其配体信号素4类蛋白A在调节性T细胞上相互作用,增强了调节性T细胞的功 能[45]。神经纤毛因子1在骨折愈合的过程中是否也接受信号素4类蛋白A的调控,而免疫系统和成骨-破骨细胞之间有何联系,有待于进一步的研究。 2.4.2 神经纤毛因子1是骨-血管偶联的关键因子 在骨组织再生过程中,骨-血管偶联是非常关键的,神经纤毛因子1也参与了骨-血管偶联。MC3T3-E1成骨细胞表达神经纤毛因子1 mRNA并与血管内皮生长因子A中的血管内皮生长因子165亚型结合。在成骨细胞中,血管内皮生长因子165亚型与成骨细胞的结合是严格依赖神经纤毛因子1的。?AB?D?-MAS?OWSKA等[46]发现间充质干细胞产生的细胞外泌体中有神经纤毛因子1等蛋白,这些细胞外泌体在体外有抑制毛细血管内皮细胞凋亡和促进血管形成、增强淋巴内皮细胞形成淋巴管的作用;他们对后肢严重缺血的小鼠采用细胞外泌体治疗后,发现小鼠损伤后肢的血管数目增加,血流得到恢复,同时淋巴管的形成也增多。但是,在骨折愈合过程中,神经纤毛因子1发挥促进骨-血管偶联的具体机制有待进一步明确。 2.4.3 其他促骨-血管偶联的因子 骨-血管偶联因子还有缺氧诱导因子1α、血小板衍生生长因子B、狭缝同源物3蛋白、鞘氨醇-1-磷酸受体、乳铁蛋白等。缺氧诱导因子1α是强大的骨-血管偶联因子,也是血小板衍生生长因子B上游的关键因子[47-49]。LIU等[50]研究发现1- 5 mmol/L的镁离子促进人脐静脉血管内皮细胞的小管形成,并且促进成骨细胞系MC3T3-E1分泌血小板衍生生长因子BB,促进成骨细胞的迁移以及碱性磷酸酶的表达,这显示血小板衍生生长因子可能是骨-血管偶联的关键因子。狭缝同源物3蛋白是小鼠模型和人类工程组织中的促血管生成因子。LI等[51]、XU等[52]研究发现狭缝同源物3蛋白来源于成骨细胞,重组的狭缝同源物3蛋白可以促进骨折愈合,可促进绝经后骨质疏松小鼠的断骨再生、减少骨质流失,这说明狭缝同源物3蛋白可能是骨-血管偶联的关键因子。ZHENG等[53]、YANG等[54]研究发现:①与野生型小鼠相比,过表达miR155的小鼠中长骨骨量低,骨折愈合缓慢;②敲除miR155的小鼠骨量高,骨折愈合迅速;③miR155作用的靶点是鞘氨醇-1-磷酸受体,激动鞘氨醇-1-磷酸受体会促进H型血管和骨再生,miR155 通过与鞘氨醇-1-磷酸受体基因的作用发挥减弱成骨和骨量的作用。鞘氨醇-1-磷酸受体在内皮细胞中分布,并且介导细胞增殖、迁移、分化和凋亡等,参与血管的形成,是重要的调节血管的因子[55],鞘氨醇-1-磷酸受体可能是骨-血管偶联的关键因子。乳铁蛋白是一种转铁蛋白,早期研究就表明它可以促进血管内皮细胞增殖和迁移、促进血管生成,最近研究发现其通过SMAD家族2/3-P38促进成骨细胞和软骨细胞的增殖和分化[56-58]。 神经纤毛因子1在骨折愈合中是否与缺氧诱导因子1α、血小板衍生生长因子B、狭缝同源物3蛋白、鞘氨醇-1-磷酸受体、乳铁蛋白等其他相关骨-血管偶联因子有联系?这些骨-血管偶联因子之间是否有网状的联系?这些骨-血管偶联因子与成骨-破骨偶联因子之间是否有联系?如何发挥作用?这些仍有待进一步的研究。 "

| [1] GBD 2019 FRACTURE COLLABORATORS. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021;2(9):e580-e592. [2] CHUCKRAN CA, LIU C, BRUNO TC, et al. Neuropilin-1: a checkpoint target with unique implications for cancer immunology and immunotherapy. J Immunother Cancer. 2020,8(2):e000967. [3] SMITH GT, RADIN DP, TSIRKA SE. From protein-protein interactions to immune modulation: Therapeutic prospects of targeting Neuropilin-1 in high-grade glioma. Front Immunol. 2022;13:958620. [4] REZAEI M, MARTINS CAVACO AC, SEEBACH J, et al. Signals of the Neuropilin-1-MET Axis and Cues of Mechanical Force Exertion Converge to Elicit Inflammatory Activation in Coherent Endothelial Cells. J Immunol. 2019;202(5):1559-1572. [5] CHIKH A, RAIMONDI C. Endothelial Neuropilin-1: a multifaced signal transducer with an emerging role in inflammation and atherosclerosis beyond angiogenesis. Biochem Soc Trans. 2024;52(1):137-150. [6] BURCKHARDT CJ, MINNA JD, DANUSER G. SH3BP4 promotes neuropilin-1 and α5-integrin endocytosis and is inhibited by Akt. Dev Cell. 2021;56(8):1164-1181.e12. [7] GRUN D, ADHIKARY G, ECKERT RL. NRP-1 interacts with GIPC1 and SYX to activate p38 MAPK signaling and cancer stem cell survival. Mol Carcinog. 2019;58(4):488-499. [8] BAYLISS AL, SUNDARARAMAN A, GRANET C, et al. Raftlin is recruited by neuropilin-1 to the activated VEGFR2 complex to control proangiogenic signaling. Angiogenesis. 2020;23(3):371-383. [9] NOAH AA, EL-MEZAYEN NS, EL-GANAINY SO, et al. Reversal of fibrosis and portal hypertension by Empagliflozin treatment of CCl4-induced liver fibrosis: Emphasis on gal-1/NRP-1/TGF-β and gal-1/NRP-1/VEGFR2 pathways. Eur J Pharmacol. 2023;959: 176066. [10] SARABIPOUR S, KINGHORN K, QUIGLEY KM, et al. Trafficking dynamics of VEGFR1, VEGFR2, and NRP1 in human endothelial cells. PLoS Comput Biol. 2024;20(2):e1011798. [11] SHIRVALILOO M. The unfavorable clinical outcome of COVID-19 in smokers is mediated by H3K4me3, H3K9me3 and H3K27me3 histone marks. Epigenomics. 2022;14(3):153-162. [12] YU Y, UCHIDA-FUKUHARA Y, WENG Y, et al. Neuropilin 1 (NRP1) Positively Regulates Adipogenic Differentiation in C3H10T1/2 Cells. Int J Mol Sci. 2023;24(8):7394. [13] ROMANO E, CHORA I, MANETTI M, et al. Decreased expression of neuropilin-1 as a novel key factor contributing to peripheral microvasculopathy and defective angiogenesis in systemic sclerosis. Ann Rheum Dis. 2016;75(8):1541-1549. [14] KILARI S, WANG Y, SINGH A, et al. Neuropilin-1 deficiency in vascular smooth muscle cells is associated with hereditary hemorrhagic telangiectasia arteriovenous malformations. JCI Insight. 2022;7(9):e155565. [15] FANTIN A, LAMPROPOULOU A, GESTRI G, et al. NRP1 Regulates CDC42 Activation to Promote Filopodia Formation in Endothelial Tip Cells. Cell Rep. 2015;11(10):1577-1590. [16] WANG Y, WANG E, ZHANG Y, et al. Neuropilin-1 maintains dimethylarginine dimethylaminohydrolase 1 expression in endothelial cells, and contributes to protection from angiotensin II-induced hypertension. FASEB J. 2019;33(1): 494-500. [17] YAMASHITA S, KUDO A, KAWAKAMI H, et al. Mechanisms of angiogenic suppression in uteri exposed to diethylstilbestrol neonatally in the mouse. Biol Reprod. 2013;88(5):116. [18] SHARMA S, EHRLICH M, ZHANG M, et al. NRP1 interacts with endoglin and VEGFR2 to modulate VEGF signaling and endothelial cell sprouting. Commun Biol. 2024;7(1):112. [19] COLOTTI G, FAILLA CM, LACAL PM, et al. Neuropilin-1 is required for endothelial cell adhesion to soluble vascular endothelial growth factor receptor 1. FEBS J. 2022;289(1):183-198. [20] XIE H, CUI Z, WANG L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20(11):1270-1278. [21] GAO B, DENG R, CHAI Y, et al. Macrophage-lineage TRAP+ cells recruit periosteum-derived cells for periosteal osteogenesis and regeneration. J Clin Invest. 2019;129(6):2578-2594. [22] MUHL L, FOLESTAD EB, GLADH H, et al. Neuropilin 1 binds PDGF-D and is a co-receptor in PDGF-D-PDGFRβ signaling. J Cell Sci. 2017; 130(8):1365-1378. [23] DHAR K, DHAR G, MAJUMDER M, et al. Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1. Mol Cancer. 2010;9:209. [24] KUSUMBE AP, RAMASAMY SK, ADAMS RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014; 507(7492):323-328. [25] XU Z, KUSUMBE AP, CAI H, et al. Type H blood vessels in coupling angiogenesis-osteogenesis and its application in bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2023;111(7): 1434-1446. [26] XU J, HE SJ, XIA TT, et al. Targeting type H vessels in bone-related diseases. J Cell Mol Med. 2024;28(4):e18123. [27] DANIEL M, SHEPPARD N, CARLOS G, et al. H Vessel Formation as a Marker for Enhanced Bone Healing in Irradiated Distraction Osteogenesis. Semin Plast Surg. 2024;38(1):31-38. [28] ZHANG D, WANG Y, ZHOU Z, et al. Role of miRNA-regulated type H vessel formation in osteoporosis. Front Endocrinol (Lausanne). 2024; 15:1394785. [29] QIN X, XI Y, JIANG Q, et al. Type H vessels in osteogenesis, homeostasis, and related disorders. Differentiation. 2023;134:20-30. [30] LIU X, ZHANG P, GU Y, et al. Type H vessels: functions in bone development and diseases. Front Cell Dev Biol. 2023;11:1236545. [31] CHOE CP. A Feasible Role of Neuropilin Signaling in Pharyngeal Pouch Formation in Zebrafish. Dev Reprod. 2023;27(3):137-147. [32] ZHOU HL, WEI MH, DI DS, et al. Association between SEMA3A signaling pathway genes and BMD/OP risk: An epidemiological and experimental study. Front Endocrinol (Lausanne). 2022;13:1014431. [33] HAYASHI M, NAKASHIMA T, YOSHIMURA N, et al. Autoregulation of Osteocyte Sema3A Orchestrates Estrogen Action and Counteracts Bone Aging. Cell Metab. 2019;29(3):627-637. [34] ZHANG L, ZHENG L, LI C, et al. Sema3a as a Novel Therapeutic Option for High Glucose-Suppressed Osteogenic Differentiation in Diabetic Osteopath. Front Endocrinol (Lausanne). 2019;10:562. [35] PAN B, ZHENG L, LIU S, et al. MiR-148a deletion protects from bone loss in physiological and estrogen-deficient mice by targeting NRP1. Cell Death Discov. 2022;8(1):470. [36] ZHENG L. Luteolin Stimulates Proliferation and Inhibits Late Differentiation of Primary Rat Calvarial Osteoblast Induced by High-dose Dexamethasone via Sema3A /NRP1/Pleixin A1. Curr Pharm Biotechnol. 2021;22(11):1538-1545. [37] AZAB E, CHANDLER KB, UDA Y, et al. Osteocytes control myeloid cell proliferation and differentiation through Gsα-dependent and -independent mechanisms. FASEB J. 2020;34(8):10191-10211. [38] ZHANG H, LU Y, WU B, et al. Semaphorin 3A mitigates lipopolysaccharide-induced chondrocyte inflammation, apoptosis and extracellular matrix degradation by binding to Neuropilin-1. Bioengineered. 2021;12(2):9641-9654. [39] STÖCKL S, REICHART J, ZBORILOVA M, et al. Semaphorin 3A-Neuropilin-1 Signaling Modulates MMP13 Expression in Human Osteoarthritic Chondrocytes. Int J Mol Sci. 2022;23(22):14180. [40] LI W, ZHANG S, ZHAO Y, et al. Revealing the Key MSCs Niches and Pathogenic Genes in Influencing CEP Homeostasis: A Conjoint Analysis of Single-Cell and WGCNA. Front Immunol. 2022;13:933721. [41] SU C, WANG H, XU L, et al. MALAT1/miR-320a in bone marrow mesenchymal stem cells function may shed light on mechanisms underlying osteoporosis. Arch Med Sci. 2021;18(6):1638-1649. [42] QIU M, XIE Y, TAN G, et al. Synovial mesenchymal stem cell-derived exosomal miR-485-3p relieves cartilage damage in osteoarthritis by targeting the NRP1-mediated PI3K/Akt pathway: Exosomal miR-485-3p relieves cartilage damage. Heliyon. 2024;10(2):e24042. [43] ZHANG Y, SU J, TENG Y, et al. Nrp1, a Neuronal Regulator, Enhances DDR2-ERK-Runx2 Cascade in Osteoblast Differentiation via Suppression of DDR2 Degradation. Cell Physiol Biochem. 2015; 36(1):75-84. [44] HAYASHI M, NAKASHIMA T, TANIGUCHI M, et al. Osteoprotection by semaphorin 3A. Nature. 2012;485(7396):69-74. [45] CHAPOVAL SP, HRITZO M, QI X, et al. Semaphorin 4A Stabilizes Human Regulatory T Cell Phenotype via Plexin B1. Immunohorizons. 2019;3(2):71-87. [46] ŁABĘDŹ-MASŁOWSKA A, VERGORI L, KĘDRACKA-KROK S, et al. Mesenchymal stem cell-derived extracellular vesicles exert pro-angiogenic and pro-lymphangiogenic effects in ischemic tissues by transferring various microRNAs and proteins including ITGa5 and NRP1. J Nanobiotechnology. 2024;22(1):60. [47] KIM JM, LIN C, STAVRE Z, et al. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells. 2020;9(9):2073. [48] LI S, SONG C, YANG S, et al. Supercritical CO2 foamed composite scaffolds incorporating bioactive lipids promote vascularized bone regeneration via Hif-1α upregulation and enhanced type H vessel formation. Acta Biomater. 2019;94:253-267. [49] LI J, CHEN X, REN L, et al. Type H vessel/platelet-derived growth factor receptor β+ perivascular cell disintegration is involved in vascular injury and bone loss in radiation-induced bone damage. Cell Prolif. 2023;56(7):e13406. [50] LIU W, GUO S, TANG Z, et al. Magnesium promotes bone formation and angiogenesis by enhancing MC3T3-E1 secretion of PDGF-BB. Biochem Biophys Res Commun. 2020;528(4):664-670. [51] LI N, INOUE K, SUN J, et al. Osteoclasts are not a source of SLIT3. Bone Res. 2020;8:11. [52] XU R, YALLOWITZ A, QIN A, et al. Targeting skeletal endothelium to ameliorate bone loss. Nat Med. 2018;24(6):823-833. [53] ZHENG Z, WU L, LI Z, et al. Mir155 regulates osteogenesis and bone mass phenotype via targeting S1pr1 gene. Elife. 2023;12:e77742. [54] YANG C, LIU Y, WANG Z, et al. Controlled mechanical loading improves bone regeneration by regulating type H vessels in a S1Pr1-dependent manner. FASEB J. 2022;36(10):e22530. [55] WANG N, LI JY, ZENG B, et al. Sphingosine-1-Phosphate Signaling in Cardiovascular Diseases. Biomolecules. 2023;13(5):818. [56] TIAN M, HAN YB, YANG GY, et al. The role of lactoferrin in bone remodeling: evaluation of its potential in targeted delivery and treatment of metabolic bone diseases and orthopedic conditions. Front Endocrinol (Lausanne). 2023;14:1218148. [57] SHI P, FAN F, CHEN H, et al. A bovine lactoferrin-derived peptide induced osteogenesis via regulation of osteoblast proliferation and differentiation. J Dairy Sci. 2020;103(5):3950-3960. [58] KIM CW, SON KN, CHOI SY, et al. Human lactoferrin upregulates expression of KDR/Flk-1 and stimulates VEGF-A-mediated endothelial cell proliferation and migration. FEBS Lett. 2006;580(18):4332-4336. [59] 李争争, 赵军伟, 罗伟, 等. 神经毡蛋白-1在创伤性颅脑损伤伴胫骨骨折愈合过程中的表达变化[J].中南大学学报(医学版), 2017,42(2):154-160. [60] MIAO HQ, SOKER S, FEINER L, et al. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146(1):233-242. [61] GROSSO A, BURGER MG, LUNGER A, et al. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front Bioeng Biotechnol. 2017;5:68. [62] RODRIGUES EM, GIOVANINI AF, RIBAS CAPM, et al. The Nervous System Development Regulator Neuropilin-1 as a Potential Prognostic Marker and Therapeutic Target in Brain Cancer. Cancers (Basel). 2023;15(20):4922. [63] MENG S, HARA T, SATO H, et al. Revealing neuropilin expression patterns in pancreatic cancer: From single‑cell to therapeutic opportunities (Review). Oncol Lett. 2024;27(3):113. [64] XING Y, QIU L, LIU D, et al. The role of smart polymeric biomaterials in bone regeneration: a review. Front Bioeng Biotechnol. 2023;11: 1240861. [65] SUN F, SUN X, WANG H, et al. Application of 3D-Printed, PLGA-Based Scaffolds in Bone Tissue Engineering. Int J Mol Sci. 2022;23(10):5831. [66] LI X, ZHU L, CHE Z, et al. Progress of research on the surface functionalization of tantalum and porous tantalum in bone tissue engineering. Biomed Mater. 2024;19(4). [67] PAPYNOV EK, SHICHALIN OO, BELOV AA, et al. CaSiO3-HAp Metal-Reinforced Biocomposite Ceramics for Bone Tissue Engineering. J Funct Biomater. 2023;14(5):259. |
| [1] | Li Zikai, Zhang Chengcheng, Xiong Jiaying, Yang Xirui, Yang Jing, Shi Haishan. Potential effects of ornidazole on intracanal vascularization in endodontic regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-7. |
| [2] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [3] | Han Haihui, Ran Lei, Meng Xiaohui, Xin Pengfei, Xiang Zheng, Bian Yanqin, Shi Qi, Xiao Lianbo. Targeting fibroblast growth factor receptor 1 signaling to improve bone destruction in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1905-1912. |
| [4] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [5] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [6] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| [7] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [8] | Wang Peiguang, Zhang Xiaowen, Mai Meisi, Li Luqian, Huang Hao. Generalized equation estimation of the therapeutic effect of floating needle therapy combined with acupoint embedding on different stages of human knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1565-1571. |
| [9] | Cai Yaohao, Lang Lyu, Li Hong. Assessing the bone mass of the residual alveolar ridge in the first molar for implant placement by cone-beam computed tomography [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1572-1577. |
| [10] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [11] | Su Xiaoyang, Chen Wenting, Fu Yidan, Zhao Yan, Lan Danfeng, Yang Qiuping. Correlation between Mer receptor tyrosine kinase and diabetic peripheral neuropathy in Sprague-Dawley rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1593-1599. |
| [12] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [13] | Li Jun, Gong Jingjing, Sun Guobin, Guo Rui, Ding Yang, Qiang Lijuan, Zhang Xiaoli, Fang Zhanhai . miR-27a-3p promotes the proliferation of human hypertrophic scar fibroblasts by regulating mitogen-activated protein kinase signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1609-1617. |
| [14] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [15] | Jing Ruyi, Chen Yingxin, Cao Lei . Prognosis of deep lamellar keratoplasty versus penetrating keratoplasty in the treatment of stromal corneal dystrophy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1626-1633. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||