Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (19): 4122-4131.doi: 10.12307/2025.069
Previous Articles Next Articles
Diagnosis and treatment of osteoarthritis with exosomes derived from different stem cells and carrying non-coding RNA
Wang Zhe, Qi Yansong, Xu Yongsheng
- Orthopedic Center (Sports Medicine Center) of Inner Mongolia Autonomous Region People’s Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China
-
Received:2024-02-21Accepted:2024-04-28Online:2025-07-08Published:2024-09-13 -
Contact:Xu Yongsheng, MD, Chief physician, Orthopedic Center (Sports Medicine Center) of Inner Mongolia Autonomous Region People’s Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China -
About author:Wang Zhe, Master candidate, Orthopedic Center (Sports Medicine Center) of Inner Mongolia Autonomous Region People’s Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China. Corresponding author: Qi Yansong, MD, Associate chief physician, Associate researcher, Orthopedic Center (Sports Medicine Center) of Inner Mongolia Autonomous Region People’s Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China -
Supported by:National Natural Science Foundation of China (General Program), No. 82172444 (to XYS)
CLC Number:
Cite this article
Wang Zhe, Qi Yansong, Xu Yongsheng. Diagnosis and treatment of osteoarthritis with exosomes derived from different stem cells and carrying non-coding RNA[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4122-4131.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
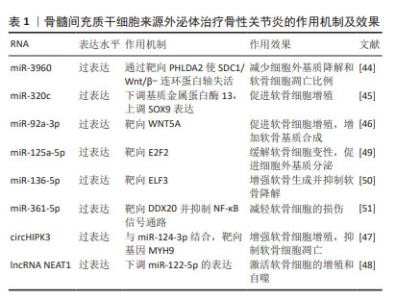
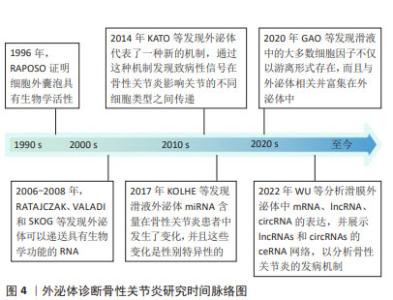
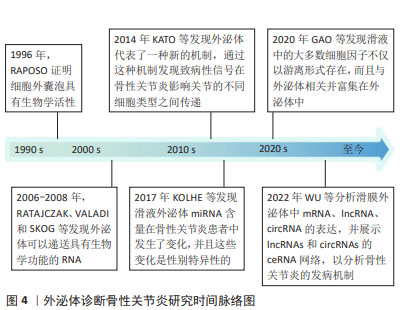
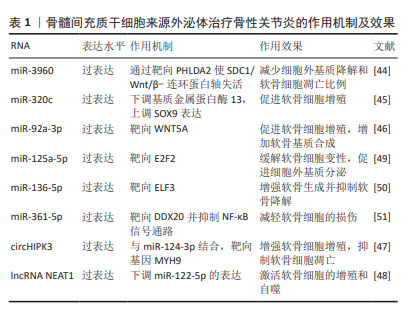
迄今为止,从血液或滑液中分离的外泌体已被确定能够有效诊断骨性关节炎[24],外泌体中的核酸,如:信使RNA(messenger RNA,mRNA)、微小RNA(microRNA,miRNA)、lncRNA、环状RNA (circular RNA,circRNA)均在骨性关节炎患者外泌体中存在差异表达。WU等[25]发现骨性关节炎组与对照组滑膜外泌体中有52个mRNA、196个lncRNA和98个circRNA表达存在差异,它们可能在骨性关节炎病理机制中发挥重要作用。骨性关节炎软骨细胞外泌体miRNA表达水平存在差异[16],骨性关节炎患者滑液中的外泌体miRNA与非骨性关节炎组存在差异并具有性别差异表达,男性与女性分别有114与144个miRNA存在表达差异[26]。 特定外泌体miRNA的表达在关节疾病患者中发生改变[27]。研究发现,破骨细胞可以通过外泌体将miRNA转移到软骨细胞上,从而改变软骨细胞的状态,降低其对软骨基质变性和血管神经侵犯的抵抗力,最终导致骨性关节炎的进一步发展[28]。KOLHE等[26]发现女性骨性关节炎患者滑液的细胞外囊泡中靶向免疫和Toll样受体相关基因的miRNA表达减少,导致预防炎症的能力降低,这可能是女性骨性关节炎发病率较高的原因之一。MENG等[29]证实,与健康受试者相比,骨性关节炎患者血浆中外泌体miR-193b-3p的表达降低。正常滑膜外泌体促进软骨形成,骨性关节炎患者成纤维细胞样滑膜细胞分泌的外泌体中miR-19b-3p,miR-19b-3p通过海绵化骨性关节炎中的溶质载体家族成员SLC7A11来加剧软骨细胞铁死亡及软骨损伤[30]。CHEN等[31]证实,相比于健康人,骨性关节炎患者滑液中外泌体miR-130b-3p和miR-1271-5p显著上调,这两种miRNA可能在骨性关节炎细胞通讯中起关键作用,具有作为标志物对骨性关节炎进行诊断的潜力。 外泌体中的一些lncRNA也具有用作骨性关节炎生物标志物的可能性。ZHAO等[32]对血浆和滑液中的外泌体lncRNA进行了分析,发现血浆来源外泌体lncRNA的表达没有显著差异,但滑液来源外泌体lncRNA前列腺特异性转录本1的表达在骨性关节炎患者中显著升高,并且在骨性关节炎的不同阶段表达存在差异,在骨性关节炎晚期表达远高于骨性关节炎早期,可用于诊断骨性关节炎的进展程度。 CircRNA是一类具有环状结构和优异稳定性非编码RNA,随着近年来基因测序技术的快速发展,circRNA的各种作用被越来越多的探索。circRNA参与骨性关节炎的一系列重要病理生理过程,尤其是其竞争性内源RNA机制,在骨性关节炎中发挥着重要作用[33]。FU等[34]发现骨性关节炎患者软骨组织中Circ_0128846表达明显上调,其通过miR-940/PTPN12通路调控骨性关节炎患者软骨细胞增殖、凋亡和炎症。血浆中circRNA-016901的表达与骨性关节炎患者疾病严重程度密切相关。骨性关节炎患者血浆中circRNA-016901的表达水平较高并在治疗后降低,而在类风湿性关节炎、骨坏死及健康对照组中未观察到血浆circRNA-016901的改变,可利用circRNA-016901在血浆中表达程度对骨性关节炎进行诊断[35]。由于circRNA内部结构高度稳定,且存在于外泌体及细胞外囊泡中,具有表达模式高度特异性和半衰期较长的特点,可作为诊断骨性关节炎的良好工具。 此外,外泌体中的蛋白质或脂类也可用作生物标志物。滑液中的细胞因子不仅游离存在,还富集于外泌体中,晚期骨性关节炎患者滑液外泌体中的细胞因子如白细胞介素1β、白细胞介素17、白细胞介素10和干扰素水平明显高于早期骨性关节炎患者[36]。KOLHE等[37]发现骨性关节炎患者外泌体中多种蛋白存在性别特异性差异,女性骨性关节炎患者外泌体中的结合珠蛋白、α-酸性糖蛋白及铜蓝蛋白的表达显著上调,男性骨性关节炎患者外泌体中β-2-糖蛋白和补体C5的表达显著上调。 外泌体在骨性关节炎早期阶段可被检测到,对于早期诊断具有重要意义。通过研究骨性关节炎患者和健康人滑液和血浆等体液来源外泌体中RNA、蛋白质及脂类等物质的差异,能够预测易感人群,外泌体有希望作为骨性关节炎早期快速诊断工具。目前外泌体对于疾病诊治处于“瓶颈期”,在未来有希望利用外泌体中所含RNA、蛋白质及脂类在骨性关节炎进展过程中发生变化的特点,实时了解病情的发展,评估骨性关节炎的治疗效果并调整制定治疗方案。 2.2 不同干细胞来源外泌体治疗骨性关节炎 2.2.1 骨髓间充质干细胞来源外泌体(bone mesenchymal stem cell-derived exosomes,BMSC-Exos) 骨髓间充质干细胞是来源于骨髓的基质干细胞,是一种具有分化为成骨、脂肪、软骨等多种分化潜能的细胞亚群。现有研究已证明,BMSC-Exos能够促进成骨和血管生成[38],同时能够极大地促进受损软骨和软骨下骨的修复[39],对骨性关节炎具有良好的治疗效果。 BMSC-Exos可以有效促进骨性关节炎软骨修复和合成。BMSC-Exos通过上调白细胞介素1β诱导的Ⅱ型胶原α1和聚集蛋白聚糖的表达以及下调基质金属蛋白酶13和含血小板反应蛋白解整合素金属肽酶5(a disintegrin and metalloproteinase with thrombospondin motifs 5,ADAMTS5)的表达,显著消除了白细胞介素1β对软骨细胞增殖和迁移的抑制作用[40]。JIN等[41]通过横断前交叉韧带和内侧半月板失稳术建立大鼠骨性关节炎模型后,在关节内注射骨髓间充质干细胞或其外泌体。BMSC-Exos能够减轻白细胞介素1β诱导的ADAMTS5、基质金属蛋白酶13和Ⅱ型胶原α1表达的改变以及通过lncRNA MEG-3抑制白细胞介素1β诱导的软骨细胞衰老和凋亡;体内实验发现,BMSC-Exos可减轻骨性关节炎大鼠关节损伤,恢复骨小梁体积分数、骨小梁数量和连接密度。骨髓间充质干细胞和BMSC-Exos均可缓解骨性关节炎大鼠软骨破坏和软骨下骨重塑,BMSC-Exos可显著抑制骨性关节炎大鼠背根神经节组织中降钙素基因相关肽和诱导型一氧化氮合酶表达的上调,从而缓解骨性关节炎的疼痛[40]。 外泌体的治疗作用主要是通过过表达各种非编码RNA完成的,包括miRNAs、 lncRNAs、circRNAs等,近年来研究人员逐渐重视对于外泌体中非编码RNA的研究。BMSC-Exos能够逆转白细胞介素1β诱导的细胞凋亡,其原因是其内含的lncRNA LYRM4-AS1通过调节GRPR-miR-6515-5p通路调控白细胞介素1β对软骨细胞的损伤从而缓解骨性关节炎炎症[42]。XU等[43]发现,BMSC-Exos可通过靶向HDAC3和STAT1/NF-κB p65,将外泌体中miR-326传递至软骨细胞和软骨,抑制软骨细胞的焦亡从而改善骨性关节炎。 BMSC-Exos能够通过多个通路影响软骨细胞的增殖、代谢及凋亡,进而促进软骨及软骨下骨的修复,起到延缓并治疗骨性关节炎的作用,并对骨性关节炎的疼痛具有良好的缓解效果。自然条件下获得的外泌体数量有限,且成分复杂多变,这限制了其在临床应用中的广泛使用。工程化外泌体直接利用细胞分泌的天然外泌体装载蛋白或者核酸等药物,通过工程化技术可以优化外泌体的载荷,使其能够高效携带和递送更多种类的治疗分子,增强对于骨性关节炎的治疗效果。 BMSC-Exos可以通过负载miR-3960来上调Ⅱ型胶原和聚集蛋白聚糖的表达,同时下调ADAMTS5和基质金属蛋白酶13的表达,以减少细胞外基质降解和软骨细胞凋亡比例[44]。SUN等[45]发现,过表达miR-320c的BMSC-Exos在促进骨关节炎软骨细胞增殖、下调基质金属蛋白酶13和上调SOX9表达方面相比未过表达特定基因的外泌体更为有效。过表达miR-92a-3p的BMSC-Exos通过靶向WNT5A,抑制WNT信号通路,促进软骨增殖,增加软骨基质合成[46]。LI等[47]证明过表达circHIPK3的BMSC-Exos可以通过与miR-124-3p结合,然后靶向基因MYH9来促进软骨细胞增殖,同时抑制软骨细胞凋亡。过表达lncRNA NEAT1能够下调miR-122-5p的表达,激活软骨细胞的增殖和自噬[48]。还有许多miRNA通过在外泌体中过表达能够有效缓解骨性关节炎,如: miR-125a-5p[49]、miR-136-5p[50]、miR-361-5p等[51]。见表1。"
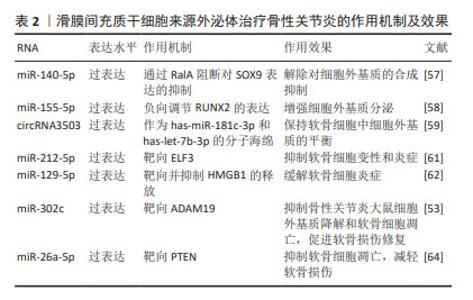
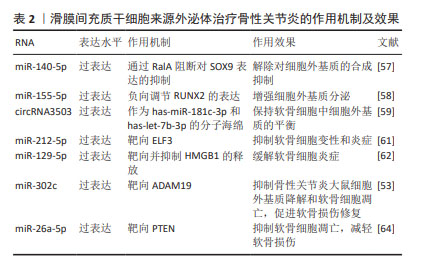
特定细胞基质的预处理也能够提高外泌体的治疗效果。脱细胞基质(decellularized extracellular matrix,dECM)为间充质干细胞的扩增提供了最佳的微环境。ZHANG等[52]发现,相比于BMSC-Exos,脱细胞基质预处理的BMSC-Exos(dECM-BMSC-Exos)靶向磷酸酯酶与张力蛋白同源物,通过上调miR-3473b的表达来促进软骨细胞的增殖、合成代谢、迁移和抗凋亡等特性,并具有更好的软骨再生效果,从而促进骨性关节炎的缓解。 BMSC-Exos能够通过多个通路影响软骨细胞的增殖、代谢及凋亡,进而促进软骨及软骨下骨的修复,起到延缓并治疗骨性关节炎的作用,并对骨性关节炎的疼痛具有良好的缓解效果。通过开发工程化外泌体携带特定RNA能够显著增强BMSC-Exos对于骨性关节炎的治疗效果。虽然BMSCs-Exos对于改善骨性关节炎具有良好的效果,但由于骨髓间充质干细胞的提取需要进行骨髓穿刺等侵入性手术,在临床应用的转化中受到一定限制。 2.2.2 滑膜间充质干细胞来源外泌体(synovial mesenchymal stem cell-derived exosomes,SMSC-Exos) 滑膜间充质干细胞能够通过传递外泌体促进软骨细胞增殖、迁移、Ⅱ型胶原α1和聚集蛋白聚糖的表达,同时抑制细胞凋亡[53]。GUO等[54]发现,SMSC-Exos可以被骨髓间充质干细胞摄取并增强其增殖及抗凋亡能力。此外,SMSC-Exos可以显著抑制地塞米松对骨髓间充质干细胞增殖的抑制作用,这表明SMSC-Exos可以用于联合治疗以提高治疗效果。 研究表明,SMSC-Exos中富含Wnt5a和Wnt5b,Wnt5a和Wnt5b通过激活Wnt信号通路,促进Yes相关蛋白的激活,从而增强软骨细胞的增殖和迁移能力。同时,Wnt5a和Wnt5b激活后抑制SOX9的表达,从而抑制细胞外基质的合成[55-56]。TAO等[57]发现,过表达miR-140-5p的SMSCs-Exos(SMSC-140-Exos)将增强关节软骨细胞的增殖和迁移能力而不会损害细胞外基质的分泌。研究人员对SMSC-Exos应用过表达miR-155-5p进行修饰,研究发现,SMSC-155-5p-Exos在体外促进骨性关节炎软骨细胞的增殖和迁移、抑制细胞凋亡,并通过结合位点负向调节Runt相关转录因子2(Runt-related transcription factor 2,Runx2)的表达增强细胞外基质分泌[58]。Runx2在骨性关节炎滑膜组织中上调,并与miR-155-5p呈负相关。体外实验证明,SMSC-155-5p-Exos在小鼠模型中可有效治疗骨性关节炎。过表达circRNA3503的SMSCs-Exos作为has-miR-181c-3p和has-let-7b-3p的分子海绵,保持软骨细胞外基质合成与降解的平衡[59]。除过表达特定RNA外,应用含脂多糖的培养基对SMSCs-Exos进行预处理也能够通过抑制细胞外基质降解,提高外泌体疗效[60]。 负载特定RNA能够增强SMSC-Exos减轻软骨细胞炎症的能力,ZHENG等[61]实验发现过表达miR-212-5p的SMSC-Exos(SMSC-212-5p-Exos)通过抑制白细胞介素1β诱导的软骨细胞中E74样ETS转录因3(E74 like ETS transcription factor 3,ELF3)的表达上调,缓解白细胞介素1β诱导的软骨细胞变性、降解和炎症反应。此外, SMSC-Exos过表达miR-129-5p也可以通过抑制HMGB1的释放来减轻白细胞介素1β诱导的骨性关节炎[62]。 外泌体也可通过过表达相应RNA改善软骨损伤。KONG等[53]研究表明,过表达miR-302c的SMSCs-Exos通过靶向去整合素和金属蛋白酶(A Disintegrin and Metallo-proteinases 19, ADAM19)促进软骨形成。过表达miR-320c的SMSCs-Exos通过降低ADAM19以及Wnt信号传导的2个关键蛋白β-catenin和MYC的水平(即靶向ADAM19依赖的Wnt信号通路),抑制骨性关节炎大鼠细胞外基质降解和软骨细胞凋亡,促进软骨损伤修复。相比于滑膜间充质干细胞及未过表达miR-320c的SMSCs-Exos可更好降低内侧半月板失稳术大鼠骨性关节炎国际研究学会(OARSI)评分,促进软骨损伤修复,抑制软骨炎症、细胞外基质降解和软骨细胞凋亡[63]。LU等[64]在体内验证了含有miR-26a-5p的SMSCs-Exos通过直接靶向PTEN基因抑制软骨凋亡及减轻软骨损伤。 针对SMSC-Exos增强骨性关节炎软骨细胞的增殖和迁移、抑制细胞凋亡,但会导致细胞外基质分泌不足的问题,研究人员利用过表达特定miRNA、circRNA或脂多糖预处理来解除合成抑制及增强合成分泌。特定RNA的过表达也能够增强SMSC-Exos减轻软骨细胞炎症、缓解软骨损伤的能力,对骨性关节炎具有更好的治疗效果。见表2。"
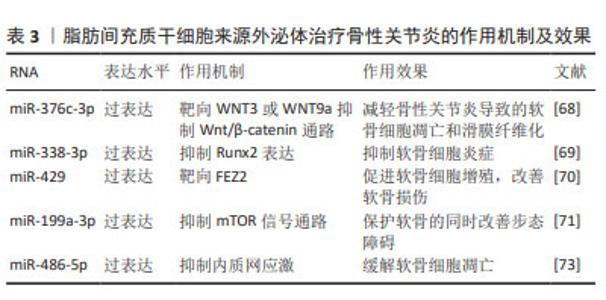
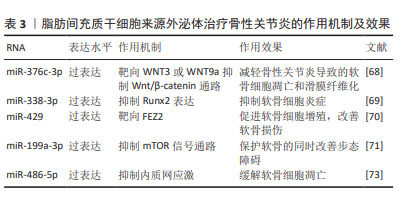
2.2.3 脂肪间充质干细胞来源外泌体(adipose mesenchymal stem cell-derived exosomes,ADSC-Exos) 脂肪间充质干细胞是来源于脂肪组织的干细胞,脂肪组织是人体最丰富的组织之一,这使得脂肪间充质干细胞具有来源丰富和获取相对容易的优势,是一种较为理想的细胞来源。脂肪间充质干细胞可以分化为软骨细胞,并分泌多种生长因子,如骨形态发生蛋白和成纤维细胞生长因子等,这些生长因子能够促进软骨再生和修复,这使得脂肪间充质干细胞成为一种治疗骨性关节炎的有效手段,关节内注射自体脂肪间充质干细胞能够显著改善骨性关节炎患者关节功能、缓解疼痛[65]。 ADSC-Exos通过旁分泌作用促进软骨再生、缓解炎症反应[66]。ZHAO等[67]研究发现ADSC-Exos通过上调miR-145和miR-221的表达,能够促进软骨形成并抑制炎症。此外,ADSC-Exos还能下调促炎标志物白细胞介素6、NF-κB和肿瘤坏死因子α的表达、上调抗炎细胞因子白细胞介素10的表达以及阻止H2O2诱导的软骨细胞凋亡。 ADSC-Exos经过修饰能达到更好地治疗骨性关节炎的效果。过表达miR-376c-3p的ADSC-Exos通过靶向WNT3或WNT9a抑制Wnt/β-catenin通路,减轻骨性关节炎导致的软骨细胞凋亡和滑膜纤维化[68]。miR-338-3p修饰的ADSC-Exos通过抑制Runx2表达可有效修复白细胞介素1β诱导的软骨细胞改变,对于骨性关节炎具有良好的缓解作用[69]。过表达miR-429的ADSC-Exos促进软骨细胞增殖,并通过靶向 FEZ2 促进自噬来改善骨关节炎的软骨损伤[70]。负载miR-199a-3p的ADSC-Exos能够促进骨性关节炎动物模型的软骨形成并抑制软骨细胞凋亡,保护软骨的同时改善步态障碍[71]。 内质网应激参与骨性关节炎中的软骨细胞凋亡和软骨退变[72]。外源性miR-486-5p可抑制内质网应激,同时能够减轻软骨细胞凋亡以及促进基质再生。WANG等[73]验证了过表达miR-486-5p的ADSC-Exos与直接使用miR-486-5p相比,前者能够更好地缓解软骨细胞凋亡以治疗骨性关节炎。 ADSC-Exos对软骨细胞的迁移和增殖具有强烈的刺激作用,同时能够很好地缓解软骨细胞凋亡及骨性关节炎软骨损伤,在人体实验中表现出良好的治疗效果。由于脂肪间充质干细胞可以通过患者特异性的方式获得,并且理论上是取之不尽的, ADSC-Exos可能是未来非常有希望应用于临床的治疗手段。见表3。"
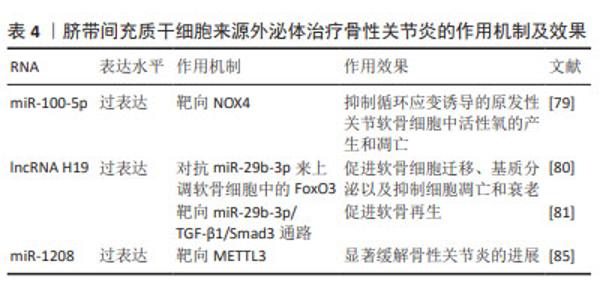
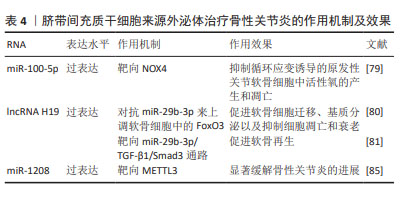
2.2.4 人脐带间充质干细胞来源外泌体(human umbilical cord mesenchymal stem cell-derived exosomes,hucMSC- Exos) 人脐带间充质干细胞由脐带组织中提取,hucMSC- Exos在促进软骨细胞增殖和迁移以及抑制软骨细胞凋亡方面有良好功效[74-75],可以逆转白细胞介素1β诱导的软骨细胞损伤,并在体外调节巨噬细胞的极化[76]。研究表明,人脐带间充质干细胞能够安全有效治疗骨性关节炎[77],并且与BMSC-Exos相比,hucMSC-Exos具有更为良好的治疗效果[78]。 LI等[79]研究发现,hucMSC-Exos通过miR-100-5p靶向NOX4,抑制循环应变诱导的原发性关节软骨细胞中活性氧的产生和凋亡。hucMSC-Exos可以作为lncRNA H19的天然载体,并且外泌体中高水平的lncRNA H19可以促进软骨细胞增殖并防止细胞凋亡[80]。YAN等[81]发现过表达lncRNA H19的hucMSC-Exos作为竞争性内源RNA对抗miR-29b-3p来上调软骨细胞中的FoxO3,促进软骨细胞迁移、基质分泌以及抑制细胞凋亡和衰老。王宪峰等[82]发现,过表达lncRNA H19的hucMSC-Exos通过靶向miR-29b-3p/TGF-β1/Smad3通路促进软骨再生。 NOD样受体热蛋白结构域相关蛋白3(NOD-like receptor thermal protein domain associated protein 3,NLRP3)是巨噬细胞炎症的重要组成部分,已被确定为骨性关节炎的潜在新型生物标志物,与诱导具有促进软骨退变和滑膜炎症功能的白细胞介素1β和白细胞介素18分泌相关[83-84]。 ZHOU等[85]证明来源于人脐带间充质干细胞的细胞外囊泡(hucMSC-EVs)通过miR-1208靶向甲基转移样酶3(methyltransferase like 3,METTL3)来抑制NLRP3 mRNA甲基化,从而抑制炎症因子释放、Ⅱ型胶原α1和聚集蛋白聚糖降解以及ADAMTS3和基质金属蛋白酶13的过表达,显著缓解小鼠膝骨性关节炎的进展。 hucMSC-Exos能够促进软骨再生,显著缓解骨性关节炎进展。hucMSCs具有易于提取且具有巨大的分化潜能的特性,脐带是临床上的医疗废物,不会对捐赠者造成伤害,因此其在治疗骨性关节炎方面具有很大的潜力。见表4。"
| [1] MARTEL-PELLETIER J, BARR AJ, CICUTTINI FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. [2] HUNTER DJ, SCHOFIELD D, CALLANDER E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014; 10(7):437-441. [3] YUE L, BERMAN J. What Is Osteoarthritis? JAMA. 2022;327(13):1300. [4] BRANDT KD, RADIN EL, DIEPPE PA, et al. Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis. 2006;65(10): 1261-1264. [5] YAO Q, WU X, TAO C, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):56. [6] CRAWFORD DC, MILLER LE, BLOCK JE. Conservative management of symptomatic knee osteoarthritis: a flawed strategy? Orthop Rev (Pavia). 2013;5(1):e2. [7] KESTER BS, MINHAS SV, VIGDORCHIK JM, et al. Total Knee Arthroplasty for Posttraumatic Osteoarthritis: Is it Time for a New Classification? J Arthroplasty. 2016;31(8):1649-1653.e1. [8] KALLURI R, LEBLEU VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [9] MATHIEU M, MARTIN-JAULAR L, LAVIEU G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9-17. [10] GYÖRGY B, SZABÓ TG, TURIÁK L, et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS One. 2012;7(11):e49726. [11] HEADLAND SE, JONES HR, NORLING LV, et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med. 2015;7(315):315ra190. [12] DOMENIS R, ZANUTEL R, CAPONNETTO F, et al. Characterization of the Proinflammatory Profile of Synovial Fluid-Derived Exosomes of Patients with Osteoarthritis. Mediators Inflamm. 2017;2017: 4814987. [13] VERGUNST CE, VAN DE SANDE MG, LEBRE MC, et al. The role of chemokines in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 2005;34(6):415-425. [14] XIE F, LIU YL, CHEN XY, et al. Role of MicroRNA, LncRNA, and Exosomes in the Progression of Osteoarthritis: A Review of Recent Literature. Orthop Surg. 2020;12(3):708-716. [15] LIU Q, WANG R, HOU S, et al. Chondrocyte-derived exosomes promote cartilage calcification in temporomandibular joint osteoarthritis. Arthritis Res Ther. 2022;24(1):44. [16] KATO T, MIYAKI S, ISHITOBI H, et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16(4):R163. [17] LU K, WANG Q, HAO L, et al. miR-204 ameliorates osteoarthritis pain by inhibiting SP1-LRP1 signaling and blocking neuro-cartilage interaction. Bioact Mater. 2023;26:425-436. [18] KAUSHIK S, CUERVO AM. Proteostasis and aging. Nat Med. 2015;21(12): 1406-1415. [19] FANG Y, NI J, WANG YS, et al. Exosomes as biomarkers and therapeutic delivery for autoimmune diseases: Opportunities and challenges. Autoimmun Rev. 2023;22(3):103260. [20] AHMAD A. Exosomes in Cancer Diagnosis and Therapy. Int J Mol Sci. 2022;23(17):9930. [21] LIU Z, LI Y, WANG Y, et al. Exosomes in HBV infection. Clin Chim Acta. 2023;538:65-69. [22] JOO HS, JEON HY, HONG EB, et al. Exosomes for the diagnosis and treatment of dementia. Curr Opin Psychiatry. 2023;36(2):119-125. [23] ALJUHANI A, ALBALAWI O, ALBALAWI R, et al. Exosomes in COVID-19 infection: Focus on role in diagnosis, pathogenesis, immunity, and clinical trials. Cell Biol Int. 2023;47(6):1049-1067. [24] YOUNG DA, BARTER MJ, SOUL J. Osteoarthritis year in review: genetics, genomics, epigenetics. Osteoarthritis Cartilage. 2022; 30(2):216-225. [25] WU X, BIAN B, LIN Z, et al. Identification of exosomal mRNA, lncRNA and circRNA signatures in an osteoarthritis synovial fluid-exosomal study. Exp Cell Res. 2022;410(1):112881. [26] KOLHE R, HUNTER M, LIU S, et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7(1):2029. [27] 孙硕,张锡光,岳乔宁,等.外泌体携载微小RNA治疗骨关节炎的研究[J].中国骨质疏松杂志,2023,29(2):248-251. [28] LIU J, WU X, LU J, et al. Exosomal transfer of osteoclast-derived miRNAs to chondrocytes contributes to osteoarthritis progression. Nat Aging. 2021;1(4):368-384. [29] MENG F, LI Z, ZHANG Z, et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics. 2018;8(10):2862-2883. [30] KONG R, JI L, PANG Y, et al. Exosomes from osteoarthritic fibroblast-like synoviocytes promote cartilage ferroptosis and damage via delivering microRNA-19b-3p to target SLC7A11 in osteoarthritis. Front Immunol. 2023;14:1181156. [31] CHEN P, RUAN A, ZHOU J, et al. Identification and analysis of key microRNAs derived from osteoarthritis synovial fluid exosomes. Chin Med J (Engl). 2023;136(2):245-247. [32] ZHAO Y, XU J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 2018; 42(12):2865-2872. [33] XUE Q, HUANG Y, CHANG J, et al. CircRNA-mediated ceRNA mechanism in Osteoarthritis: Special emphasis on circRNAs in exosomes and the crosstalk of circRNAs and RNA methylation. Biochem Pharmacol. 2023; 212:115580. [34] FU G, YIN F, ZHAO J. Depletion of circ_0128846 ameliorates interleukin-1β-induced human chondrocyte apoptosis and inflammation through the miR-940/PTPN12 pathway. Int Immunopharmacol. 2022;110:108996. [35] DU M, FAN S, LIU Y, et al. The Application of circRNA-016901 in Improving the Diagnostic Accuracy of Osteoarthritis. Biomed Res Int. 2022;2022:1158562. [36] GAO K, ZHU W, LI H, et al. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod Rheumatol. 2020;30(4):758-764. [37] KOLHE R, OWENS V, SHARMA A, et al. Sex-Specific Differences in Extracellular Vesicle Protein Cargo in Synovial Fluid of Patients with Osteoarthritis. Life (Basel). 2020;10(12):337. [38] ZHANG L, JIAO G, REN S, et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11(1):38. [39] ASGHAR S, LITHERLAND GJ, LOCKHART JC, et al. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology (Oxford). 2020;59(1):57-68. [40] HE L, HE T, XING J, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11(1):276. [41] JIN Y, XU M, ZHU H, et al. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J Cell Mol Med. 2021;25(19):9281-9294. [42] WANG X, LI Z, CUI Y, et al. Exosomes Isolated From Bone Marrow Mesenchymal Stem Cells Exert a Protective Effect on Osteoarthritis via lncRNA LYRM4-AS1-GRPR-miR-6515-5p. Front Cell Dev Biol. 2021;9:644380. [43] XU H, XU B. BMSC-Derived Exosomes Ameliorate Osteoarthritis by Inhibiting Pyroptosis of Cartilage via Delivering miR-326 Targeting HDAC3 and STAT1//NF-κB p65 to Chondrocytes. Mediators Inflamm. 2021;2021:9972805. [44] YE P, MI Z, WEI D, et al. miR-3960 from Mesenchymal Stem Cell-Derived Extracellular Vesicles Inactivates SDC1/Wnt/β-Catenin Axis to Relieve Chondrocyte Injury in Osteoarthritis by Targeting PHLDA2. Stem Cells Int. 2022;2022:9455152. [45] SUN H, HU S, ZHANG Z, et al. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J Cell Biochem. 2019;120(1):171-181. [46] MAO G, ZHANG Z, HU S, et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 2018;9(1):247. [47] LI S, LIU J, LIU S, et al. Chitosan oligosaccharides packaged into rat adipose mesenchymal stem cells-derived extracellular vesicles facilitating cartilage injury repair and alleviating osteoarthritis. J Nanobiotechnology. 2021;19(1):343. [48] ZHANG S, JIN Z. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Containing Long Noncoding RNA NEAT1 Relieve Osteoarthritis. Oxid Med Cell Longev. 2022;2022:5517648. [49] XIA Q, WANG Q, LIN F, et al. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered. 2021;12(2):11225-11238. [50] CHEN X, SHI Y, XUE P, et al. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res Ther. 2020;22(1):256. [51] TAO Y, ZHOU J, WANG Z, et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorg Chem. 2021;113:104978. [52] ZHANG Y, QI G, YAN Y, et al. Exosomes derived from bone marrow mesenchymal stem cells pretreated with decellularized extracellular matrix enhance the alleviation of osteoarthritis through miR-3473b/phosphatase and tensin homolog axis. J Gene Med. 2023;25(8):e3510. [53] KONG R, GAO J, ZHANG J, et al. Synovial mesenchymal stem cell-derived exosomal miR-320c enhances chondrogenesis by targeting ADAM19. Future Med Chem. 2022;14(2):81-96. [54] GUO SC, TAO SC, YIN WJ, et al. Exosomes from Human Synovial-Derived Mesenchymal Stem Cells Prevent Glucocorticoid-Induced Osteonecrosis of the Femoral Head in the Rat. Int J Biol Sci. 2016;12(10):1262-1272. [55] PARK HW, KIM YC, YU B, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162(4):780-794. [56] LIU CF, LEFEBVRE V. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis. Nucleic Acids Res. 2015;43(17):8183-8203. [57] TAO SC, YUAN T, ZHANG YL, et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180-195. [58] WANG Z, YAN K, GE G, et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol Toxicol. 2021;37(1):85-96. [59] TAO SC, HUANG JY, GAO Y, et al. Small extracellular vesicles in combination with sleep-related circRNA3503: A targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact Mater. 2021;6(12):4455-4469. [60] DUAN A, SHEN K, LI B, et al. Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell Res Ther. 2021;12(1):427. [61] ZHENG T, LI Y, ZHANG X, et al. Exosomes Derived From miR-212-5p Overexpressed Human Synovial Mesenchymal Stem Cells Suppress Chondrocyte Degeneration and Inflammation by Targeting ELF3. Front Bioeng Biotechnol. 2022;10:816209. [62] QIU M, LIU D, FU Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269:118987. [63] KONG R, ZHANG J, JI L, et al. Synovial mesenchymal stem cell-derived exosomal microRNA-320c facilitates cartilage damage repair by targeting ADAM19-dependent Wnt signalling in osteoarthritis rats. Inflammopharmacology. 2023;31(2):915-926. [64] LU L, WANG J, FAN A, et al. Synovial mesenchymal stem cell-derived extracellular vesicles containing microRN555A-26a-5p ameliorate cartilage damage of osteoarthritis. J Gene Med. 2021;23(11):e3379. [65] LEE WS, KIM HJ, KIM KI, et al. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl Med. 2019;8(6):504-511. [66] TOFIÑO-VIAN M, GUILLÉN MI, PÉREZ DEL CAZ MD, et al. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxid Med Cell Longev. 2017;2017:7197598. [67] ZHAO C, CHEN JY, PENG WM, et al. Exosomes from adipose‑derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR‑145 and miR‑221. Mol Med Rep. 2020;21(4): 1881-1889. [68] LI F, XU Z, XIE Z, et al. Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microRNA -376c-3p and targeting the WNT-beta-catenin signaling axis. Apoptosis. 2023; 28(3-4):362-378. [69] LI C, LI W, PU G, et al. Exosomes derived from miR-338-3p-modified adipose stem cells inhibited inflammation injury of chondrocytes via targeting RUNX2 in osteoarthritis. J Orthop Surg Res. 2022;17(1):567. [70] MENG C, NA Y, HAN C, et al. Exosomal miR-429 derived from adipose-derived stem cells ameliorated chondral injury in osteoarthritis via autophagy by targeting FEZ2. Int Immunopharmacol. 2023;120:110315. [71] ZHAO S, XIU G, WANG J, et al. Engineering exosomes derived from subcutaneous fat MSCs specially promote cartilage repair as miR-199a-3p delivery vehicles in Osteoarthritis. J Nanobiotechnology. 2023;21(1):341. [72] LIU Y, ZHU H, YAN X, et al. Endoplasmic reticulum stress participates in the progress of senescence and apoptosis of osteoarthritis chondrocytes. Biochem Biophys Res Commun. 2017;491(2):368-373. [73] WANG Y, FAN A, LU L, et al. Exosome modification to better alleviates endoplasmic reticulum stress induced chondrocyte apoptosis and osteoarthritis. Biochem Pharmacol. 2022;206:115343. [74] MIANEHSAZ E, MIRZAEI HR, MAHJOUBIN-TEHRAN M, et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10(1):340. [75] 郭天赐,刘爱峰,陈继鑫,等.人脐带源间充质干细胞源性外泌体在骨科疾病中的研究进展[J].河北医药,2023,45(1):125-130. [76] LI P, LV S, JIANG W, et al. Exosomes derived from umbilical cord mesenchymal stem cells protect cartilage and regulate the polarization of macrophages in osteoarthritis. Ann Transl Med. 2022;10(18):976. [77] 孙月,沈阳,刘铭,等.人脐带血间充质干细胞治疗膝关节小范围软骨缺损疗效研究[J].创伤与急危重病医学,2022,10(3):191-194. [78] 王宪峰,欧昕,邓必勇.不同来源间充质干细胞外泌体治疗骨关节炎疗效的比较[J].中国组织工程研究,2022,26(25):3980-3985. [79] LI X, WANG Y, CAI Z, et al. Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR-100-5p/NOX4 axis. Cell Biol Int. 2021;45(10):2096-2106. [80] YAN L, LIU G, WU X. Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J Orthop Translat. 2020;26:111-120. [81] YAN L, LIU G, WU X. The umbilical cord mesenchymal stem cell-derived exosomal lncRNA H19 improves osteochondral activity through miR-29b-3p/FoxO3 axis. Clin Transl Med. 2021;11(1):e255. [82] 王宪峰,王锟,孙晗,等.脐带间充质干细胞外泌体LncRNA H19修复软骨损伤的机制[J].中国组织工程研究,2024,28(1):20-25. [83] SWANSON KV, DENG M, TING JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477-489. [84] JIN C, FRAYSSINET P, PELKER R, et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci U S A. 2011;108(36):14867-14872. [85] ZHOU H, SHEN X, YAN C, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate osteoarthritis of the knee in mice model by interacting with METTL3 to reduce m6A of NLRP3 in macrophage. Stem Cell Res Ther. 2022;13(1):322. [86] FU Y, CUI S, ZHOU Y, et al. Dental Pulp Stem Cell-Derived Exosomes Alleviate Mice Knee Osteoarthritis by Inhibiting TRPV4-Mediated Osteoclast Activation. Int J Mol Sci. 2023;24(5):4926. [87] LIN T, WU N, WANG L, et al. Inhibition of chondrocyte apoptosis in a rat model of osteoarthritis by exosomes derived from miR‑140‑5p‑overexpressing human dental pulp stem cells. Int J Mol Med. 2021;47(3):7. [88] BERETTI F, ZAVATTI M, CASCIARO F, et al. Amniotic fluid stem cell exosomes: Therapeutic perspective. Biofactors. 2018;44(2):158-167. [89] ZAVATTI M, BERETTI F, CASCIARO F, et al. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. Biofactors. 2020;46(1):106-117. [90] JIANG B, FU X, YAN L, et al. Transplantation of human ESC-derived mesenchymal stem cell spheroids ameliorates spontaneous osteoarthritis in rhesus macaques. Theranostics. 2019;9(22): 6587-6600. [91] ZHANG S, CHU WC, LAI RC, et al. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135-2140. [92] WANG Y, YU D, LIU Z, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017; 8(1):189. [93] KO JY, IM GI. Chondrogenic and Osteogenic Induction from iPS Cells. Methods Mol Biol. 2016;1357:441-450. [94] LIU X, LI Q, NIU X, et al. Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis. Int J Biol Sci. 2017;13(2):232-244. [95] ZHU Y, WANG Y, ZHAO B, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8(1):64. [96] LIU Y, ZENG Y, SI HB, et al. Exosomes Derived From Human Urine-Derived Stem Cells Overexpressing miR-140-5p Alleviate Knee Osteoarthritis Through Downregulation of VEGFA in a Rat Model. Am J Sports Med. 2022;50(4):1088-1105. [97] WANG Y, ZHANG C, WANG N, et al. Genetic basis of ruminant headgear and rapid antler regeneration. Science. 2019;364(6446):eaav6335. [98] WANG D, BERG D, BA H, et al. Deer antler stem cells are a novel type of cells that sustain full regeneration of a mammalian organ-deer antler. Cell Death Dis. 2019;10(6):443. [99] LEI J, JIANG X, LI W, et al. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell. 2022;13(3):220-226. |
| [1] | Li Jiagen, Chen Yueping, Huang Keqi, Chen Shangtong, Huang Chuanhong. The construction and validation of a prediction model based on multiple machine learning algorithms and the immunomodulatory analysis of rheumatoid arthritis from the perspective of mitophagy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-15. |
| [2] | Zhang Hao, Wang Qing, Zhang Jian, Li Guangzhou, Wang Gaoju. Comparison of posterior C2-3 fixation combined with bucking bar technique and posterior C2-3 fixation alone in treatment of unstable Hangman fractures [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1848-1854. |
| [3] | Su Lintao, Jiang Jianfeng, Ma Jun, Huang Liangliang, Lei Changyu, Han Yaozheng, Kang Hui. Precise application of O-arm navigation system in thoracolumbar fractures with developmental pedicle stenosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1855-1862. |
| [4] | Ma Chi, Wang Ning, Chen Yong, Wei Zhihan, Liu Fengji, Piao Chengzhe. Application of 3D-printing patient-specific instruments combined with customized locking plate in opening wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1863-1869. |
| [5] | Gao Zhenyang, Zeng Xiuan, Yang Qibing, Kou Xianshuai, Wang Kejing, Li Meng. Computer-simulated repositioning combined with pelvic reduction frame for treatment of anteroposterior compression-III pelvic fractures [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1870-1875. |
| [6] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [7] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [8] | Sun Yundi, Cheng Lulu, Wan Haili, Chang Ying, Xiong Wenjuan, Xia Yuan. Effect of neuromuscular exercise for knee osteoarthritis pain and function: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1945-1952. |
| [9] | Deng Keqi, Li Guangdi, Goswami Ashutosh, Liu Xingyu, He Xiaoyong. Screening and validation of Hub genes for iron overload in osteoarthritis based on bioinformatics [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1972-1980. |
| [10] | Li Liangkui, Huang Yongcan, Wang Peng, Yu Binsheng. Effect of anterior controllable anteriodisplacement and fusion on vertebrae-ossification of posterior longitudinal ligament complex and implants: a finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1761-1767. |
| [11] | Chen Xi, Tang Tao, Chen Tongbing, Li Qing, Zhang Wen. Mechanical stability of intertrochanteric fracture of femur with different internal fixation systems [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1783-1788. |
| [12] | Huang Haobo, Liang Xinyuan, Ye Guozhong, Xie Qingxiang, Su Boyuan. Suture tape and headless compression screws in treatment of Lisfranc injury with comminuted fractures of the first and second proximal metatarsal bones [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1803-1809. |
| [13] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [14] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [15] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||