Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (5): 888-898.doi: 10.12307/2024.593
Previous Articles Next Articles
Naringin inhibits iron deposition and cell apoptosis in bone tissue of osteoporotic rats
Lan Shuangli1, Xiang Feifan2, Deng Guanghui2, Xiao Yukun2, Yang Yunkang2, Liang Jie2
- 1Department of Hepatobiliary Surgery, 2Department of Bone and Joint Surgery, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China
-
Received:2023-08-04Accepted:2023-12-14Online:2025-02-18Published:2024-06-01 -
Contact:Liang Jie, Master, Attending physician, Department of Bone and Joint Surgery, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China -
About author:Lan Shuangli, Department of Hepatobiliary Surgery, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China -
Supported by:Joint Innovation Special Project of Sichuan Science and Technology Program, No. 2022YFS0628 (to XFF); Natural Fund Project of Sichuan Provincial Science and Technology Department, No. 2022NSFSC1534 (to YYK)
CLC Number:
Cite this article
Lan Shuangli, Xiang Feifan, Deng Guanghui, Xiao Yukun, Yang Yunkang, Liang Jie. Naringin inhibits iron deposition and cell apoptosis in bone tissue of osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 888-898.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
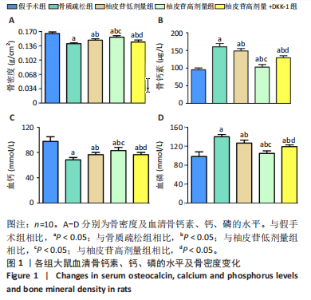
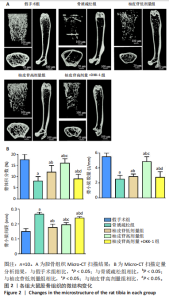
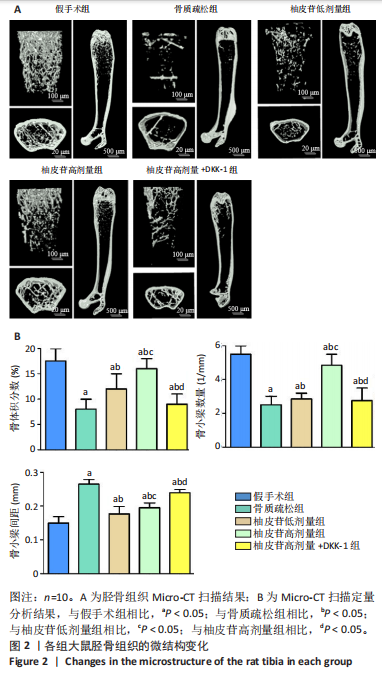
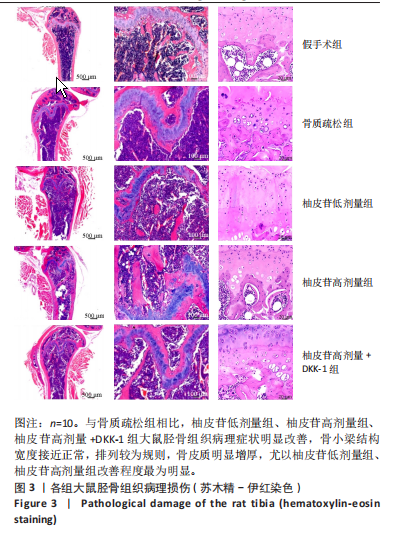
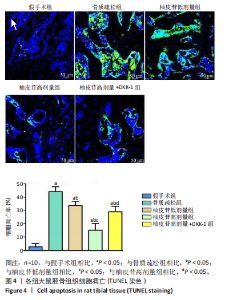
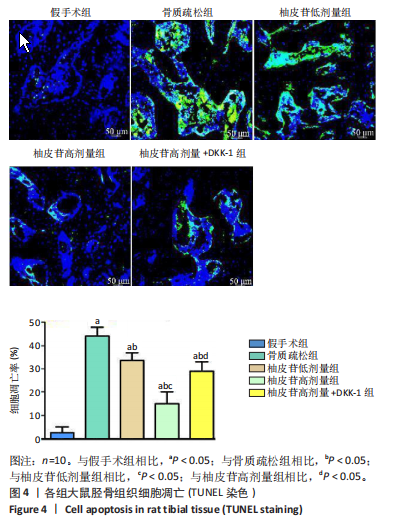
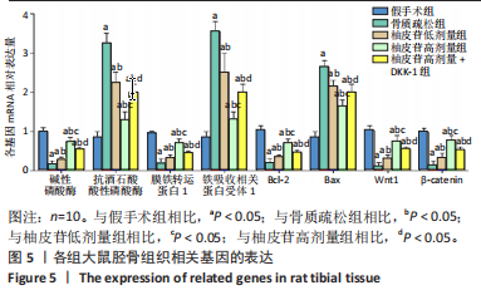
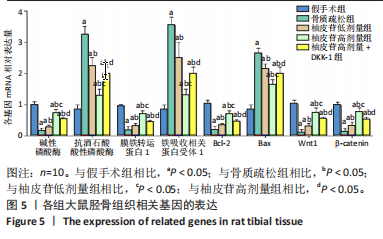
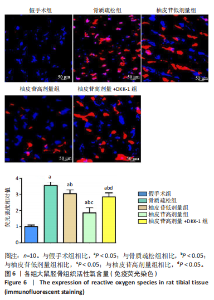
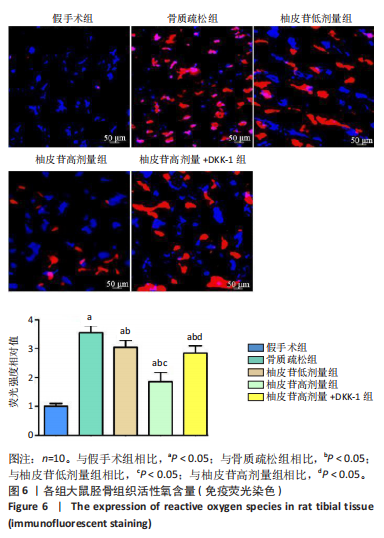
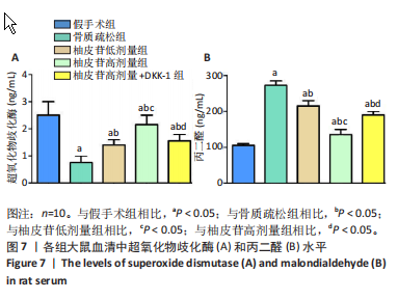
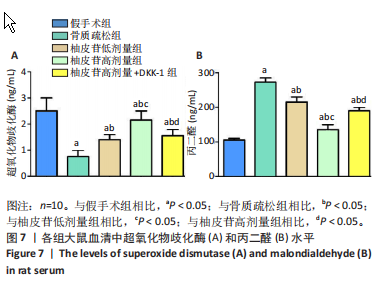
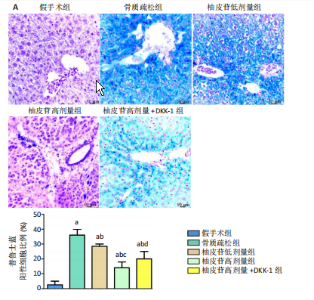
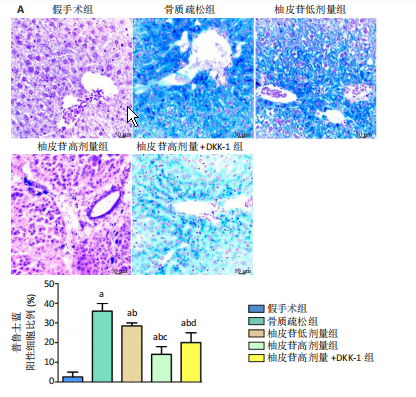
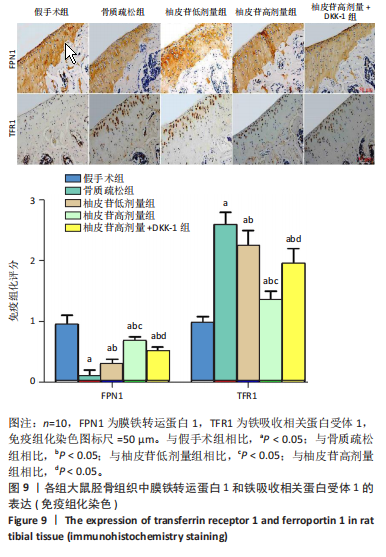
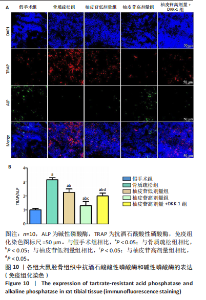
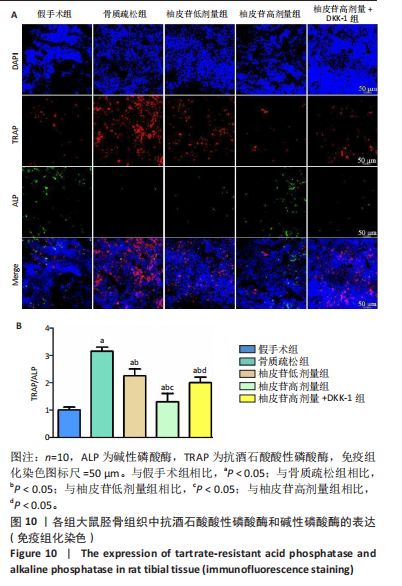
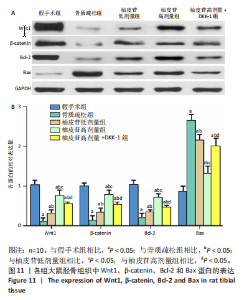
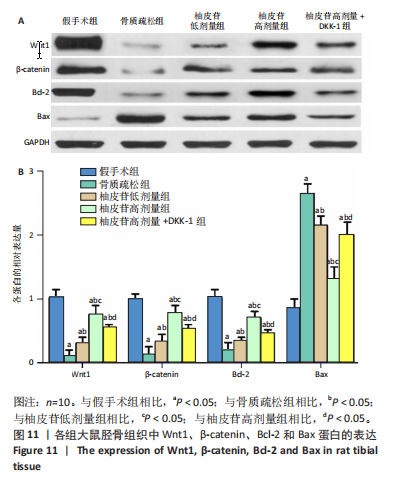
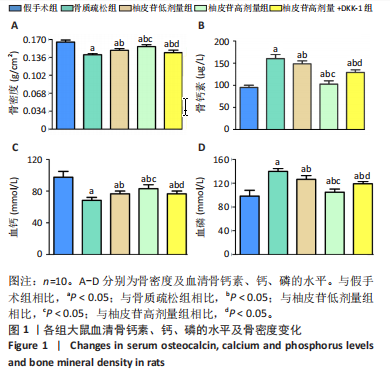
2.1 各组大鼠造模结果及一般表现 在实验过程中未发生动物死亡的现象。造模后,假手术组大鼠活泼活动,反应机敏,皮毛顺滑浓密,摄食、饮水及睡眠未见异常;骨质疏松组大鼠精神状态偏于狂躁不安,反应迟钝,皮毛暗淡稀疏,大鼠在实验过程中往往处于少动嗜睡的状态,摄食与饮水意愿不强烈;柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组大鼠的情绪、精神状态较为稳定,反应较为敏捷,皮毛较为顺整。 与假手术组相比,骨质疏松组大鼠血清钙水平和骨密度明显下降(P < 0.05),血清骨钙素和磷的水平明显升高(P < 0.05),证实造模成功。 2.2 各组大鼠血清中骨钙素、钙、磷水平及骨密度的变化 与假手术组相比,骨质疏松组、柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组大鼠血清钙水平和骨密度下降(P < 0.05),骨钙素和磷水平升高(P < 0.05);与骨质疏松组相比,柚皮苷干预组大鼠血清钙水平和骨密度升高(P < 0.05),骨钙素和磷水平下降(P < 0.05);与柚皮苷高剂量组相比,柚皮苷低剂量组、柚皮苷高剂量+DKK-1组大鼠血清钙水平和骨密度下降(P < 0.05),骨钙素和磷水平升高(P < 0.05),见图1。 2.3 各组大鼠骨微结构的变化 Micro-CT扫描结果显示,与假手术组相比,骨质疏松组、柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组大鼠胫骨组织的平均骨体积分数和平均骨小梁数下降(P < 0.05),骨小梁间距升高(P < 0.05);与骨质疏松组相比,柚皮苷干预组大鼠胫骨组织的平均骨体积分数和平均骨小梁数升高(P < 0.05),骨小梁间距下降(P < 0.05);与柚皮苷高剂量组相比,柚皮苷低剂量组、柚皮苷高剂量+DKK-1组大鼠胫骨组织的平均骨体积分数和平均骨小梁数下降(P < 0.05),骨小梁间距升高(P < 0.05),见图2。 2.4 各组大鼠骨组织病理损伤 苏木精-伊红染色结果显示,假手术组大鼠胫骨组织形态正常,结构完整,骨小梁粗细匀称,排列致密有序,骨小梁内纹理清晰可辨,骨皮质厚度均一,未见明显异常;骨质疏松组大鼠胫骨组织结构破坏明显,骨小梁数量减少,排列紊乱,结构变细,模糊不清晰,部分有断裂、缺失现象,髓腔扩张明显,骨皮质变薄,出现部分空洞结构;与骨质疏松组相比,柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组大鼠胫骨组织病理症状明显改善,骨小梁结构宽度接近正常,排列较为规则,骨皮质明显增厚,尤以柚皮苷低剂量组、柚皮苷高剂量组改善程度最为明显,见图3。 2.5 各组大鼠胫骨组织细胞凋亡情况 胫骨组织TUNEL染色显示,与假手术组相比,骨质疏松组、柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组细胞凋亡率升高(P < 0.05);与骨质疏松组相比,柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组细胞凋亡率下降(P < 0.05);与柚皮苷高剂量组相比,柚皮苷低剂量组、柚皮苷高剂量+DKK-1组细胞凋亡率升高(P < 0.05),见图4。 2.6 各组大鼠胫骨组织中相关基因的表达 胫骨组织PCR检测显示,与假手术组相比,骨质疏松组、柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组抗酒石酸酸性磷酸酶、铁吸收相关蛋白受体1、Bax的mRNA表达升高(P < 0.05),碱性磷酸酶、膜铁转运蛋白1、Bcl-2、Wnt1、β-catenin的mRNA表达降低(P < 0.05);与骨质疏松组相比,柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组抗酒石酸酸性磷酸酶、铁吸收相关蛋白受体1、Bax的mRNA表达降低(P < 0.05),碱性磷酸酶、膜铁转运蛋白1、Bcl-2、Wnt1、β-catenin的mRNA表达升高(P < 0.05);与柚皮苷高剂量组相比,柚皮苷低剂量组、柚皮苷高剂量+DKK-1组抗酒石酸酸性磷酸酶、铁吸收相关蛋白受体1、Bax的mRNA表达升高(P < 0.05),碱性磷酸酶、膜铁转运蛋白1、Bcl-2、Wnt1、β-catenin的mRNA表达降低(P < 0.05),见图5。 2.7 各组大鼠胫骨组织中氧化应激水平 胫骨组织超氧化物阴离子二氢乙啶染色显示,与假手术组相比,骨质疏松组、柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组大鼠活性氧含量升高(P < 0.05);与骨质疏松组相比,柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组活性氧含量降低(P < 0.05);与柚皮苷高剂量组相比,柚皮苷低剂量组、柚皮苷高剂量+DKK-1组活性氧含量升高(P < 0.05),见图6。 与假手术组相比,骨质疏松组、柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组大鼠血清丙二醛水平升高(P < 0.05),超氧化物歧化酶水平下降(P < 0.05);与骨质疏松组相比,柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组大鼠血清丙二醛水平下降(P < 0.05),超氧化物歧化酶水平升高(P < 0.05);与柚皮苷高剂量组相比,柚皮苷低剂量组、柚皮苷高剂量+DKK-1组大鼠血清丙二醛水平明显升高(P < 0.05),超氧化物歧化酶水平下降(P < 0.05),见图7。 2.8 各组大鼠胫骨组织和肝组织中铁沉积情况 普鲁士蓝染色显示,与假手术组相比,骨质疏松组、柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组大鼠肝组织与骨组织中普鲁士蓝阳性细胞比例升高(P < 0.05);与骨质疏松组相比,柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组大鼠肝组织与骨组织中中普鲁士蓝阳性细胞的比例降低(P < 0.05);与柚皮苷高剂量组相比,柚皮苷低剂量组、柚皮苷高剂量+DKK-1组大鼠肝组织与骨组织中普鲁士蓝阳性细胞比例升高(P < 0.05),见图8。 胫骨组织免疫组化染色显示,与假手术组相比,骨质疏松组、柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组膜铁转运蛋白1的表达降低(P < 0.05),铁吸收相关蛋白受体1的表达升高(P < 0.05);与骨质疏松组相比,柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组膜铁转运蛋白1的表达升高(P < 0.05),铁吸收相关蛋白受体1的表达降低(P < 0.05);与柚皮苷高剂量组相比,柚皮苷低剂量组、柚皮苷高剂量+DKK-1组膜铁转运蛋白1的表达降低(P < 0.05),铁吸收相关蛋白受体1的表达升高(P < 0.05),见图9。 2.9 各组大鼠胫骨组织中抗酒石酸酸性磷酸酶和碱性磷酸酶的表达 胫骨组织免疫荧光染色显示,与假手术组相比,骨质疏松组、柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组碱性磷酸酶表达明显降低(P < 0.05),抗酒石酸酸性磷酸酶表达升高(P < 0.05);与骨质疏松组相比,柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组碱性磷酸酶表达升高(P < 0.05),抗酒石酸酸性磷酸酶表达明显降低(P < 0.05);与柚皮苷高剂量组相比,柚皮苷低剂量组、柚皮苷高剂量+DKK-1组碱性磷酸酶表达降低(P < 0.05),抗酒石酸酸性磷酸酶表达升高(P < 0.05),见图10。 2.10 各组大鼠胫骨组织中Wnt1信号通路相关蛋白的表达 胫骨组织Western blot检测显示,与假手术组相比,骨质疏松组、柚皮苷低剂量组、柚皮苷高剂量组、柚皮苷高剂量+DKK-1组Bcl-2、Wnt1、β-catenin蛋白表达降"
| [1] PATEL D, WAIRKAR S. Bone regeneration in osteoporosis: opportunities and challenges. Drug Deliv Transl Res. 2023;13(2):419-432. [2] LAIRD C, BENSON H, WILLIAMS KA. Pharmacist interventions in osteoporosis management: a systematic review. Osteoporos Int. 2023; 34(2):239-254. [3] CHAKRABARTI K, MCCUNE WJ. Glucocorticoid-induced osteoporosis in premenopausal women: management for the rheumatologist. Curr Opin Rheumatol. 2023;35(3):161-169. [4] ZHANG G, ZHEN C, YANG J, et al. 1-2 T static magnetic field combined with Ferumoxytol prevent unloading-induced bone loss by regulating iron metabolism in osteoclastogenesis. J Orthop Translat. 2022;38(11):126-140. [5] VASIKARAN SD, MIURA M, PIKNER R, et al. Practical Considerations for the Clinical Application of Bone Turnover Markers in Osteoporosis. Calcif Tissue Int. 2023;112(2):148-157. [6] LI J, SONG J, MENG D, et al. Electrospun naringin-loaded microsphere/sucrose acetate isobutyrate system promotes macrophage polarization toward M2 and facilitates osteoporotic bone defect repair. Regen Biomater. 2023;2(10):1-16. [7] HU J, LIN X, GAO P, et al. Genotypic and phenotypic spectrum and pathogenesis of WNT1 variants in a large cohort of patients with OI/osteoporosis. J Clin Endocrinol Metab. 2023;3(1):1-11. [8] MOUSAVI S, VAKILI S, ZAL F, et al. Quercetin potentiates the anti-osteoporotic effects of alendronate through modulation of autophagy and apoptosis mechanisms in ovariectomy-induced bone loss rat model. Mol Biol Rep. 2023;50(4):3693-3703. [9] HWANG JH, PARK YS, KIM HS, et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J Control Release. 2023;355(3):184-198. [10] CHEN M, FU W, XU H, et al. Pathogenic mechanisms of glucocorticoid-induced osteoporosis. Cytokine Growth Factor Rev. 2023;70(4):54-66. [11] ZHANG K, QIU W, LI H, et al. MACF1 overexpression in BMSCs alleviates senile osteoporosis in mice through TCF4/miR-335-5p signaling pathway. J Orthop Translat. 2023;39(3):177-190. [12] ZHUANG Q, CHEN S, ZHANG W, et al. Avicularin Alleviates Osteoporosis in Ovariectomized Mice by Inhibiting Osteoclastogenesis through NF-κB Pathway Inhibition. J Agric Food Chem. 2023;71(1):411-420. [13] XIE H, CAO L, YE L, et al. microRNA-29b-3p/sirtuin-1/peroxisome proliferator-activated receptor γ suppress osteogenic differentiation. In Vitro Cell Dev Biol Anim. 2023;59(2):109-120. [14] LEE AS, SUNG MJ, SON SJ, et al. Effect of Menaquinone-4 on Receptor Activator of Nuclear Factor κB Ligand-Induced Osteoclast Differentiation and Ovariectomy-Induced Bone Loss. J Med Food. 2023;26(2):128-134. [15] ZENG C, WANG S, CHEN F, et al. Alpinetin alleviates osteoporosis by promoting osteogenic differentiation in BMSCs by triggering autophagy via PKA/mTOR/ULK1 signaling. Phytother Res. 2023;37(1):252-270. [16] BEEKMAN KM, DUQUE G, CORSI A, et al. Osteoporosis and Bone Marrow Adipose Tissue. Curr Osteoporos Rep. 2023;21(1):45-55. [17] QASEEM A, HICKS LA, ETXEANDIA-IKOBALTZETA I, et al. Pharmacologic Treatment of Primary Osteoporosis or Low Bone Mass to Prevent Fractures in Adults: A Living Clinical Guideline From the American College of Physicians. Ann Intern Med. 2023;176(2):224-238. [18] GE X, JIANG F, WANG M, et al. Naringin@Metal-Organic Framework as a Multifunctional Bioplatform. ACS Appl Mater Interfaces. 2023; 15(1):677-683. [19] CHENG C, PENG X, XI L, et al. Feasibility study of oxidized naringin as a novel crosslinking agent for crosslinking decellularized porcine Achilles tendon and its potential application for anterior cruciate ligament repair. J Biomed Mater Res A. 2023;111(2):170-184. [20] ZHENG L, SHEN X, XIE Y, et al. Metformin promotes osteogenic differentiation and prevents hyperglycaemia-induced osteoporosis by suppressing PPARγ expression. Acta Biochim Biophys Sin (Shanghai). 2023;55(3):394-403. [21] WANG M, LI H, TANG J, et al. Effect of simvastatin on osteogenesis of the extremity bones in aging rats. Connect Tissue Res. 2023;64(1): 64-74. [22] WU H, ZHANG D, XIA H, et al. SDH5 down-regulation mitigates the damage of osteoporosis via inhibiting the MyD88/NF-κB signaling pathway. Immunopharmacol Immunotoxicol. 2023;45(3):317-327. [23] WAWRZYNIAK N, GRAMAZA-MICHALOWSKA A, KURZAWA P, et al. Calcium carbonate-enriched pumpkin affects calcium status in ovariectomized rats. J Food Sci Technol. 2023;60(4):1402-1413. [24] 张敏,张晓明,刘童斌.柚皮苷在骨组织再生领域的应用潜力[J].中国组织工程研究,2023,27(5):787-792. [25] GAN J, DENG X, LE Y, et al. The Development of Naringin for Use against Bone and Cartilage Disorders. Molecules. 2023;28(9):3716-3736. [26] LIU Y, ZHU S, LIU J, et al. Vitexin Regulates Angiogenesis and Osteogenesis in Ovariectomy-Induced Osteoporosis of Rats via the VDR/PI3K/AKT/eNOS Signaling Pathway. J Agric Food Chem. 2023; 71(1):546-556. [27] SUN H, XU J, WANG Y, et al. Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair. Bioact Mater. 2023;24(1):477-496. [28] HE Q, YANG J, PAN Z, et al. Biochanin A protects against iron overload associated knee osteoarthritis via regulating iron levels and NRF2/System xc-/GPX4 axis. Biomed Pharmacother. 2023;157:113915-113930. [29] FENG M, LIU L, WANG J, et al. The Molecular Mechanisms Study of Engeletin Suppresses RANKL-Induced Osteoclastogenesis and Inhibits Ovariectomized Murine Model Bone Loss. J Inflamm Res. 2023;16(5):2255-2270. [30] NOONAN ML, NI P, SOLIS E, et al. Osteocyte Egln1/Phd2 links oxygen sensing and biomineralization via FGF23. Bone Res. 2023;11(1):7-23. [31] JIN C, TAN K, YAO Z, et al. A Novel Anti-Osteoporosis Mechanism of VK2: Interfering with Ferroptosis via AMPK/SIRT1 Pathway in Type 2 Diabetic Osteoporosis. J Agric Food Chem. 2023;71(6):2745-2761. [32] MENG D, SONG J, YI Y, et al. Controlled released naringin-loaded liposome/sucrose acetate isobutyrate hybrid depot for osteogenesis in vitro and in vivo. Front Bioeng Biotechnol. 2023;10(1):1097178-1097190. [33] ZHANG H, WANG A, LI G, et al. Osteoporotic bone loss from excess iron accumulation is driven by NOX4-triggered ferroptosis in osteoblasts. Free Radic Biol Med. 2023;198(3):123-136. [34] BAE S, KIM K, KANG K, et al. RANKL-responsive epigenetic mechanism reprograms macrophages into bone-resorbing osteoclasts. Cell Mol Immunol. 2023;20(1):94-109 [35] CHEN R, WANG M, QI Q, et al. Sequential anti-inflammatory and osteogenic effects of a dual drug delivery scaffold loaded with parthenolide and naringin in periodontitis. J Periodontal Implant Sci. 2023;53(1):20-37. [36] ZHANG W, ZHOU X, HOU W, et al. Reversing the imbalance in bone homeostasis via sustained release of SIRT-1 agonist to promote bone healing under osteoporotic condition. Bioact Mater. 2022;19(5): 429-443. [37] AYERS C, KANGSAGARA D, LAZUR B, et al. Effectiveness and Safety of Treatments to Prevent Fractures in People With Low Bone Mass or Primary Osteoporosis: A Living Systematic Review and Network Meta-analysis for the American College of Physicians. Ann Intern Med. 2023;176(2):182-195. [38] OH WT, YANG YS, XIE J, et al. WNT-modulating gene silencers as a gene therapy for osteoporosis, bone fracture, and critical-sized bone defects. Mol Ther. 2023; 31(2):435-453. [39] WANG X, MA Y, CHEN J, et al. A novel decellularized matrix of Wnt signaling-activated osteocytes accelerates the repair of critical-sized parietal bone defects with osteoclastogenesis, angiogenesis, and neurogenesis. Bioact Mater. 2022; 21(8):110-128. [40] FU M, TIAN Y, ZHANG T, et al. Comparative study of DHA-enriched phosphatidylcholine and EPA-enriched phosphatidylcholine on ameliorating high bone turnover via regulation of the osteogenesis-related Wnt/β-catenin pathway in ovariectomized mice. Food Funct. 2020;11(11):10094-10104. |
| [1] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [2] | Zhou Jinhai, Li Jiangwei, Wang Xuquan, Zhuang Ying, Zhao Ying, Yang Yuyong, Wang Jiajia, Yang Yang, Zhou Shilian. Three-dimensional finite element analysis of anterior femoral notching during total knee arthroplasty at different bone strengths [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1775-1782. |
| [3] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [4] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [5] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [6] | Li Yueyao, Zhang Min, Yang Jiaju. Cistanoside A mediates p38/MAPK pathway to inhibit osteoclast activity [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1144-1151. |
| [7] | Zheng Lin, Jin Wenjun, Luo Shanshan, Huang Rui, Wang Jie, Cheng Yuting, An Zheqing, Xiong Yue, Gong Zipeng, Liao Jian. Eucommia ulmoides promotes alveolar bone formation in ovariectomized rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1159-1167. |
| [8] |
Huang Xiaobin, Ge Jirong, Li Shengqiang, Xie Lihua, Huang Jingwen, He Yanyan, Xue Lipeng.
Mechanisms of different yin nourishing and kidney tonifying methods on osteoclastysis pathway in ovariectomized rats #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1214-1219.
|
| [9] | Qian Kun, Li Ziqing, Sun Shui . Endoplasmic reticulum stress in the occurrence and development of common degenerative bone diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1285-1295. |
| [10] | Wang Dongyang, Yang Qiaohui, Lin Xinchao. Relationship between vitamin D levels and reproductive characteristics and exercise dietary situation in postmenopausal women [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1021-1025. |
| [11] | Zhang Lichuang, Yang Wen, Ding Guangjiang, Li Peikun, Xiao Zhongyu, Chen Ying, Fang Xue, Zhang Teng. Dispersion effect of bone cement after vertebroplasty using individualized unilateral external pedicle approach and bilateral pedicle approach [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 800-808. |
| [12] | Zhang Xiongjinfu, Chen Yida, Cheng Xinyi, Liu Daihui, Shi Qin . Exosomes derived from bone marrow mesenchymal stem cells of young rats to reverse senescence in aged rat bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7709-7718. |
| [13] | Yang Lei, Liu Sanmao, Sun Huanwei, Che Chao, Tang Lin. Comparison of six machine learning models suitable for use in medicine: support for osteoporosis screening and initial diagnosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7499-7510. |
| [14] | Liu Xuan, Ding Yuqing, Xia Ruohan, Wang Xianwang, Hu Shujuan. Exercise prevention and treatment of insulin resistance: role and molecular mechanism of Keap1/nuclear factor erythroid2-related factor 2 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7578-7588. |
| [15] | Yan Jing, Qin Qiujun, Li Fen, Zhou Jun, Ding Yuanyuan, Jin Chunlin. A systematic review of osteoporosis-specific quality-of-life scales [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7649-7655. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||