Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (4): 761-770.doi: 10.12307/2025.216
Previous Articles Next Articles
Visualization analysis of research hotspots of artificial intelligence in field of spinal cord nerve injury and repair
Yang Bin1, Tao Guangyi2, Yang Shun1, Xu Junjie2, Huang Junqing1
- 1Department of Pain, Henan Province Hospital of TCM/Second Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China; 2College of Bone Injury, Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2023-12-01Accepted:2024-01-15Online:2025-02-08Published:2024-05-31 -
Contact:Huang Junqing, Master’s supervisor, Chief physician, Department of Pain, Henan Province Hospital of TCM/Second Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
About author:Yang Bin, Master, Associate chief physician, Department of Pain, Henan Province Hospital of TCM/Second Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
Supported by:National TCM Clinical Characteristics Technology Inheritance Talent Project (National TCM Education Letter (2019) No. 36) (to YB); Henan Province Physical Education Research Project, No. 202320 (to YB)
CLC Number:
Cite this article
Yang Bin, Tao Guangyi, Yang Shun, Xu Junjie, Huang Junqing . Visualization analysis of research hotspots of artificial intelligence in field of spinal cord nerve injury and repair[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 761-770.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

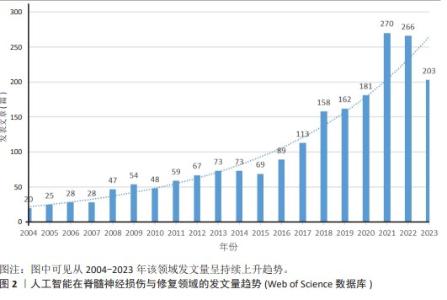
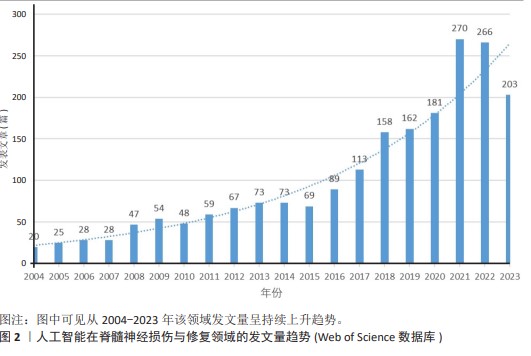
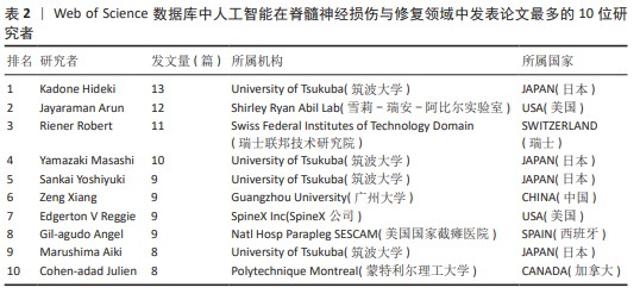
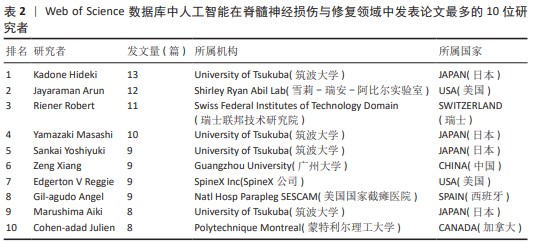
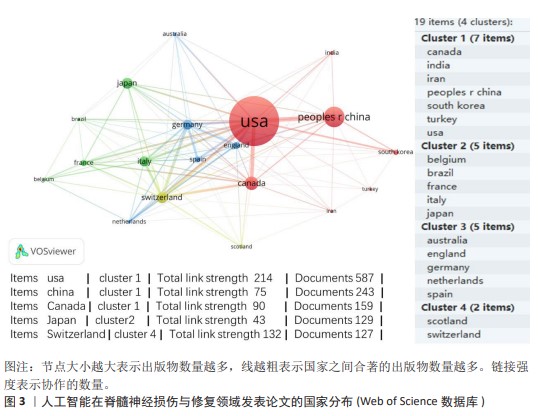
2.2 国家分析和作者分析 VOSviewer 1.6.19软件检测结果表明,有19个国家至少有24篇关于该研究主题的出版物,按研究方向划分为4个聚类,见图3。发表论文最多的5个国家是美国、中国、加拿大、日本和瑞士,这些国家在人工智能在脊髓神经损伤与修复领域的研究成果最多。Kadone Hideki(University of Tsukuba,JAPAN)、Jayaraman Arun(Shirley Ryan Abil Lab,USA)和Riener Robert(Swiss Federal Institutes of Technology Domain,SWITZERLAND)等作者在这一领域的研究最为活跃,见表2。"

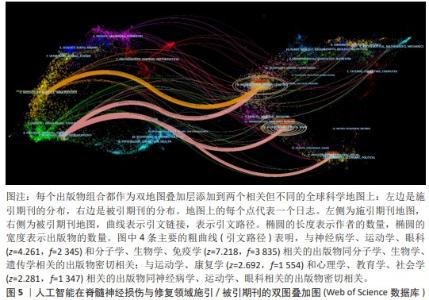
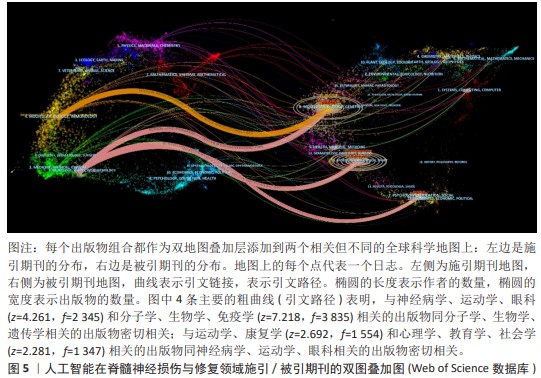
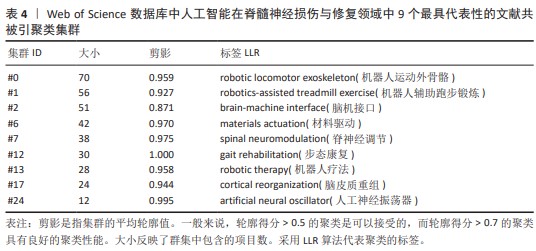
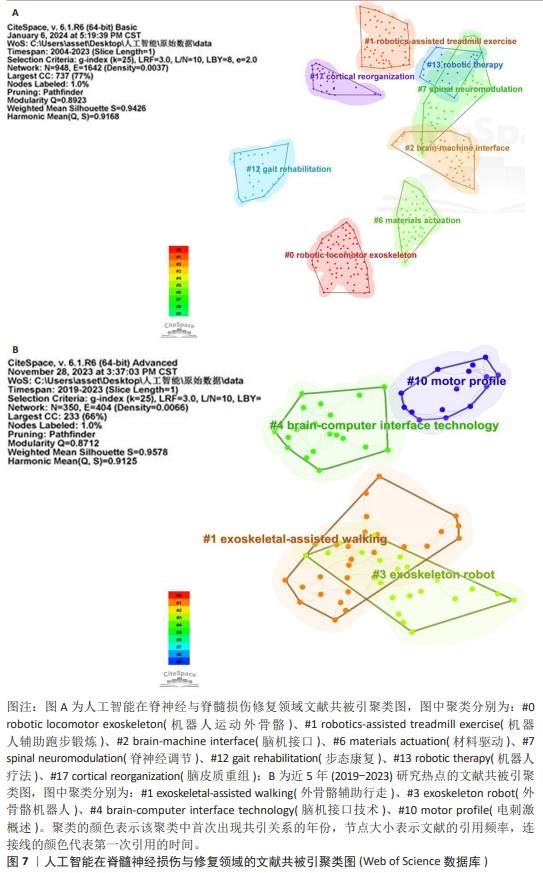
2.5 文献共被引分析 2.5.1 文献共被引聚类分析 总共获得了25个集群,去除与检索词相近的聚类后,获得文献共被引网络中最显著的9个集群,见图7A与表4。 集群#0,#6:机器运动外骨骼、材料驱动。这两个集群均是聚焦于机器外骨骼,主要引用文章是EVANS等[8](2015)和SANCHEZ-VILLAMA?AN等[9](2019)。近年来,机器人运动外骨骼的开发取得了重大进展,为脊髓损伤患者提供站立和行走的机会,也为步态辅助和康复提供了相当多的解决方案。该领域重点关注3个方面:驱动、结构和接口连接组件。 集群#1,#12:机器人辅助步态训练。这两个集群主要引用文章是BANALA等[10](2009)和FANG等[11](2020)。步态训练对于促进神经肌肉可塑性至关重要,这是改善功能性行走能力所必需的。机器人辅助步态训练是为使用主动腿部外骨骼和力场控制器的脊髓损伤患者开发的,力场控制器通过髋关节和膝关节上的致动器有效地在受试者的脚踝处施加力来实现康复作用。 集群#2:脑机接口。该集群中的主要引用文章是COLLINGER等[12](2013),上肢瘫痪或截肢导致失去上肢抓握、操纵和搬运物体的能力,这些功能对日常生活活动至关重要。脑机接口可以为恢复许多失去的功能提供解决方案。文章在患者运动皮质中植入了2个96通道皮质内微电极,测试了四肢瘫痪患者可以使用这种脑机接口快速实现对高性能假肢的神经控制。 集群#7:脊神经调节。该集群中的主要引用文章是ANGELI等[13](2014)文章,结果证明通过硬膜外刺激对脊柱回路进行神经调节,使完全瘫痪的个体能够处理概念、听觉和视觉输入,以重新获得对瘫痪肌肉的相对精细的自主控制;腰骶脊髓网络兴奋性亚阈值运动状态的神经调控是被诊断为腿部完全瘫痪的个体中恢复有意识运动的关键。发现了一种全新的干预策略,即使在受伤多年后,也可以显著影响完全瘫痪个体的自主运动恢复。 集群#13:机器人疗法。该集群中的主要引用文章是CORTES等[14](2013)文章,主要探讨恢复创伤性脊髓损伤后四肢瘫痪患者的上肢运动功能。也表明机器人辅助设备是量化运动性能(运动学)的绝佳工具,可用于灵敏地测量给定康复干预后的变化。 集群#17:脑皮质重组。该集群中的主要引用文章是COURTINE等[15](2009)文章,主要探讨失去大脑输入后将无功能的脊髓回路转化为功能状态,研究发现在完全脊髓横断去除成年大鼠所有脊髓上输入后,血清素能激动剂和硬膜外电刺激的组合能够在受伤后1周将脊柱网络从无功能状态急性转变为高功能和适应性状态。这为脊髓损伤患者重新获得高水平的运动控制提供了一种策略。 集群#24:人工神经振荡器。该集群中的主要引用文章是IJSPEERT等[16](2007)研究,提出了一个脊髓模型及其在两栖蝾螈机器人中的实现,该脊髓模型展示了原始神经回路如何通过肢体振荡中心进行扩展,证明肢体振荡中心比身体振荡中心具有更低的固有频率,这为脊神经与脊髓损伤患者重新获得高水平的运动控制提供了一种策略。"
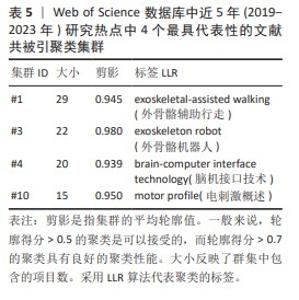
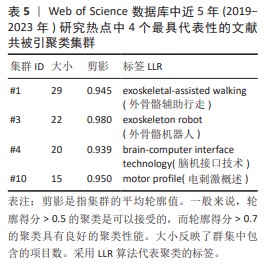
2.5.2 近5年研究热点的共被引文献聚类分析 总共获得了11个集群,去除与检索词相近的聚类后,获得包括文献共被引网络中最显著的4个集群。这4个最具代表性的集群详见图7B与表5。 集群#1,#3:机器人运动外骨骼辅助运动与康复。这两个集群均是聚焦于机器外骨骼,主要引用文章是FANG等[11](2020)和SANCHEZ- VILLAMA?AN等[9](2019)。近年来,机器人运动外骨骼的开发取得了重大进展,为脊髓损伤患者提供站立和行走的机会,也为步态辅助和康复提供了相当多的解决方案。该领域重点关注3个方面:驱动、结构和接口连接。 集群#4:脑机接口技术。该集群中的主要引用文章是AJIBOYE等[17](2017),可以通过对周围肌肉和神经进行协调电刺激(又称功能性电刺激)来恢复慢性四肢瘫痪患者的肢体运动;通过植入的功能性电刺激组件和皮质内脑机接口,可使用患者自身的皮质信号指挥肢体运动。这是首例联合植入的功能性电刺激+皮质内脑机接口神经假体,代表了临床上神经假体可行性的重大进展。 集群#10:电刺激概述。该集群中的主要引用文章是GILL等[18](2018),由于脊髓损伤而在功能上与大脑断开的脊髓感觉运动网络可以通过硬膜外电刺激来促进瘫痪患者恢复强大、协调的运动活动。在存在硬膜外电刺激的情况下进行动态任务训练,文章称为多模式康复。该文是第一份因脊髓损伤导致下肢感觉、运动功能丧失的患者进行上述多模式康复的报告。"

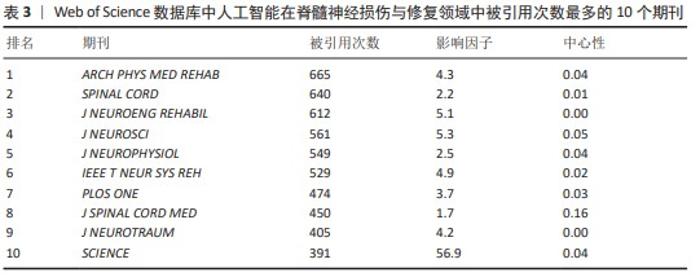
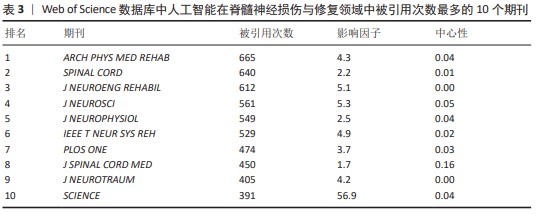
2.5.3 高共被引文献热点分析 对高共被引文献展开深入分析,采用CiteSpace 6.1.R6软件对人工智能在脊髓神经损伤与修复领域从建库至2023年共被引次数前10位的文献进行分析[19-28],结果见表6。这些文献均为该领域的核心文献,具有较强的学术影响力,能够反映该领域的研究热点与前沿,为相关研究提供重要的参考。 排名第一的高被引文献为MILLER等[19](2016),共被引次数为73次。该文章首次对已发表的动力外骨骼治疗脊髓损伤患者研究的临床有效性和安全性进行荟萃分析,发现随着新一代外骨骼的出现和对患者资格标准的改进,痉挛状态、跌倒等严重不良事件的发生率得到明显缓解;此文章共被引程度最高,反映了动力外骨骼在人工智能治疗脊髓损伤和修复领域受到诸多学者的认可。同时LOUIE等[21](2015),ZEILIG等[25](2012)及HARTIGAN等[27](2015)均进行了机器外骨骼治疗脊髓损伤患者的荟萃分析,主要研究内容为系统评估机器外骨骼的安全性、耐受性和行走功能功效等,从不同角度证明了机器外骨骼治疗脊髓损伤患者的优越性。 剩余6篇排名前10位的高共被引文献均为临床研究,研究内容包括多种动力外骨骼、不同分型的脊髓损伤患者(完全性胸椎脊髓损伤、慢性运动不完全性脊髓损伤和异质性脊髓损伤等)、多种运动训练方式等,研究结果均证明了动力外骨骼可显著恢复脊髓损伤患者的功能性行走和运动能力,其安全性也得到了验证。"

| [1] BICAN O, MINAGAR A, PRUITT AA. The spinal cord: a review of functional neuroanatomy. Neurol Clin. 2013;31(1):1-18. [2] ELI I, LERNER DP, GHOGAWALA Z. Acute traumatic spinal cord injury. Neurol Clin. 2021;39(2):471-488. [3] LUU DK, NGUYEN AT, JIANG M, et al. Deep learning-based approaches for decoding motor intent from peripheral nerve signals. Front Neurosci. 2021;15:667907. [4] LUU DK, NGUYEN AT, JIANG M, et al. Artificial intelligence enables real-time and intuitive control of prostheses via nerve interface. IEEE Trans Biomed Eng. 2022;69(10):3051-3063. [5] ROMEO-GUITART D, FORéS J, HERRANDO-GRABULOSA M, et al. Neuroprotective drug for nerve trauma revealed using artificial intelligence. Sci Rep. 2018;8(1):1879. [6] GUO Y, SUN L, ZHONG W, et al. Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges. Neural Regen Res. 2024;19(3):663-670. [7] GOLDBERG D, MCCOUCH S, KLEINBERG J. Constructing comparative genome maps with unresolved marker order. Pac Symp Biocomput. 2002;7:139-150. [8] EVANS N, HARTIGAN C, KANDILAKIS C, et al. Acute cardiorespiratory and metabolic responses during exoskeleton-assisted walking overground among persons with chronic spinal cord injury. Top Spinal Cord Inj Rehabil. 2015;21(2):122-132. [9] SANCHEZ-VILLAMAñAN MDC, GONZALEZ-VARGAS J, TORRICELLI D, et al. Compliant lower limb exoskeletons: a comprehensive review on mechanical design principles. J Neuroeng Rehabil. 2019;16(1):55. [10] BANALA SK, KIM SH, AGRAWAL SK, et al. Robot assisted gait training with active leg exoskeleton (ALEX). IEEE Trans Neural Syst Rehabil Eng. 2009;17(1):2-8. [11] FANG CY, TSAI JL, LI GS, et al. Effects of robot-assisted gait training in individuals with spinal cord injury: a meta-analysis. Biomed Res Int. 2020;2020:2102785. [12] COLLINGER JL, WODLINGER B, DOWNEY JE, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381(9866):557-564. [13] ANGELI CA, EDGERTON VR, GERASIMENKO YP, et al. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014; 137(Pt 5):1394-1409. [14] CORTES M, ELDER J, RYKMAN A, et al. Improved motor performance in chronic spinal cord injury following upper-limb robotic training. Neurorehabilitation. 2013; 33(1):57-65. [15] COURTINE G, GERASIMENKO Y, VAN DEN BRAND R, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12(10):1333-1342. [16] IJSPEERT AJ, CRESPI A, RYCZKO D, et al. From swimming to walking with a salamander robot driven by a spinal cord model. Science. 2007;315(5817): 1416-1420. [17] AJIBOYE AB, WILLETT FR, YOUNG DR, et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet. 2017;389(10081):1821-1830. [18] GILL ML, GRAHN PJ, CALVERT JS, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018; 24(11):1677-1682. [19] MILLER LE, ZIMMERMANN AK, HERBERT WG. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: systematic review with meta-analysis. Med Devices (Auckl). 2016;9:455-466. [20] ESQUENAZI A, TALATY M, PACKEL A, et al. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am J Phys Med Rehabil. 2012; 91(11):911-921. [21] LOUIE DR, ENG JJ, LAM T. Gait speed using powered robotic exoskeletons after spinal cord injury: a systematic review and correlational study. J Neuroeng Rehabil. 2015;12:82. [22] WIRZ M, ZEMON DH, RUPP R, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil. 2005;86(4):672-680. [23] BACH BAUNSGAARD C, VIG NISSEN U, KATRIN BRUST A, et al. Gait training after spinal cord injury: safety, feasibility and gait function following 8 weeks of training with the exoskeletons from Ekso Bionics. Spinal Cord. 2018;56(2):106-116. [24] KOZLOWSKI AJ, BRYCE TN, DIJKERS MP. Time and effort required by persons with spinal cord injury to learn to use a powered exoskeleton for assisted walking. Top Spinal Cord Inj Rehabil. 2015;21(2):110-121. [25] ZEILIG G, WEINGARDEN H, ZWECKER M, et al. Safety and tolerance of the ReWalk™ exoskeleton suit for ambulation by people with complete spinal cord injury: a pilot study. J Spinal Cord Med. 2012;35(2): 96-101. [26] NAM KY, KIM HJ, KWON BS, et al. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: a systematic review. J Neuroeng Rehabil. 2017;14(1):24. [27] HARTIGAN C, KANDILAKIS C, DALLEY S, et al. Mobility outcomes following five training sessions with a powered exoskeleton. Top Spinal Cord Inj Rehabil. 2015;21(2):93-99. [28] FIELD-FOTE EC, ROACH KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther. 2011;91(1):48-60. [29] HOMCHANTHANAKUL J, MANOONPONG P. Continuous online adaptation of bioinspired adaptive neuroendocrine control for autonomous walking robots. IEEE Trans Neural Netw Learn Syst. 2022; 33(5):1833-1845. [30] QI W, FAN H, KARIMI HR, et al. An adaptive reinforcement learning-based multimodal data fusion framework for human-robot confrontation gaming. Neural Netw. 2023; 164:489-496. [31] PIGOZZI F, MEDVET E. Evolving modularity in soft robots through an embodied and self-organizing neural controller. Artif Life. 2022; 28(3):322-347. [32] TAN R, GAO L, KHAN N, et al. Interpretable Artificial Intelligence through Locality Guided Neural Networks. Neural Netw. 2022;155:58-73. [33] SRISUCHINNAWONG A, HOMCHANTHANAKUL J, MANOONPONG P. NeuroVis: real-time neural information measurement and visualization of embodied neural systems. Front Neural Circuits. 2021;15:743101. [34] GORRE N, CARRANZA E, FUHRMAN J, et al. MIDRC CRP10 AI interface-an integrated tool for exploring, testing and visualization of AI models. Phys Med Biol. 2023;68(7):10. [35] JIANG Y, WANG Y, MIAO Z, et al. Composite-learning-based adaptive neural control for dual-arm robots with relative motion. IEEE Trans Neural Netw Learn Syst. 2022; 33(3):1010-1021. [36] LI X, ZHANG T, LI C, et al. Electrical stimulation accelerates Wallerian degeneration and promotes nerve regeneration after sciatic nerve injury. Glia. 2023;71(3):758-774. [37] FISHER LE, LEMPKA SF. Neurotechnology for pain. Annu Rev Biomed Eng. 2023;25: 387-412. [38] DOLBOW DR, GORGEY AS, JOHNSTON TE, et al. Electrical stimulation exercise for people with spinal cord injury: a healthcare provider perspective. J Clin Med. 2023; 12(9):3150. [39] BARRA B, CONTI S, PERICH MG, et al. Epidural electrical stimulation of the cervical dorsal roots restores voluntary upper limb control in paralyzed monkeys. Nat Neurosci. 2022;25(7):924-934. [40] LUO S, RABBANI Q, CRONE NE. Brain-computer interface: applications to speech decoding and synthesis to augment communication. Neurotherapeutics. 2022; 19(1):263-273. [41] CAJIGAS I, VEDANTAM A. Brain-computer interface, neuromodulation, and neurorehabilitation strategies for spinal cord injury. Neurosurg Clin N Am. 2021;32(3):407-417. [42] COLUCCI A, VERMEHREN M, CAVALLO A, et al. Brain-computer interface-controlled exoskeletons in clinical neurorehabilitation: ready or not? Neurorehabil Neural Repair. 2022;36(12):747-756. [43] BOUTON CE. Merging brain-computer interface and functional electrical stimulation technologies for movement restoration. Handb Clin Neurol. 2020;168: 303-309. [44] STAMPACCHIA G, GAZZOTTI V, OLIVIERI M, et al. Gait robot-assisted rehabilitation in persons with spinal cord injury: a scoping review. Neurorehabilitation. 2022; 51(4):609-647. [45] MIGALEV AS, VIGASINA KD, GOTOVTSEV PM. A review of motor neural system robotic modeling approaches and instruments. Biol Cybern. 2022;116(3):271-306. [46] TRAN P, JEONG S, WOLF SL, et al. Patient-specific, voice-controlled, robotic flexotendon glove-ii system for spinal cord injury. IEEE Robot Autom Lett. 2020;5(2): 898-905. [47] OGATA K, UMINO T, NAKAYAMA T, et al. Dummy humanoid robot simulating several trunk postures and abdominal shapes - Report of element technologies. Advanced Robotics. 2017;31(6):303-310. [48] SHE HT, ZHANG WM, HUANG HL, et al. Anti-skid foot design for a humanoid robot[C]//2014 IEEE International Conference on Robotics and Biomimetics (ROBIO 2014).[2024-02-03]. [49] KOVACIC J, CANAGASINGHAM A, GOOLAM A, et al. A robot-assisted laparoscopic revision of an artificial urinary sphincter in a patient with spinal cord injury. BJU Int. 2023. doi: 10.1111/bju.16153. [50] LIU DD, HE JQ, SINHA R, et al. Purification and characterization of human neural stem and progenitor cells. Cell. 2023;186(6): 1179-1194.e1115. [51] SOTO J, DING X, WANG A, et al. Neural crest-like stem cells for tissue regeneration. Stem Cells Transl Med. 2021;10(5):681-693. [52] SHENDE P, DEVLEKAR NP. A review on the role of artificial intelligence in stem cell therapy: an initiative for modern medicines. Curr Pharm Biotechnol. 2021;22(9):1156-1163. [53] LI G, ZHANG B, SUN JH, et al. An NT-3-releasing bioscaffold supports the formation of TrkC-modified neural stem cell-derived neural network tissue with efficacy in repairing spinal cord injury. Bioact Mater. 2021;6(11):3766-3781. [54] YUAN T, SHAO Y, ZHOU X, et al. Highly permeable DNA supramolecular hydrogel promotes neurogenesis and functional recovery after completely transected spinal cord injury. Adv Mater. 2021;33(35):e2102428. [55] BUENO A, BOSCH I, RODRíGUEZ A, et al. Automated cervical spinal cord segmentation in real-world MRI of multiple sclerosis patients by optimized hybrid residual attention-aware convolutional neural networks. J Digit Imaging. 2022; 35(5):1131-1142. [56] CHEN G, WU H, CHEN N, et al. Potential of intraoperative ultrasonographic assessment of the spinal cord central echo complex in predicting postoperative neurological recovery of degenerative cervical myelopathy. Eur J Neurol. 2022;29(1): 217-224. [57] CARSON T, GHOSHAL G, CORNWALL GB, et al. Artificial intelligence-enabled, real-time intraoperative ultrasound imaging of neural structures within the psoas: validation in a porcine spine model. Spine (Phila Pa 1976). 2021;46(3):E146-E152. [58] NOZAWA K, MAKI S, FURUYA T, et al. Magnetic resonance image segmentation of the compressed spinal cord in patients with degenerative cervical myelopathy using convolutional neural networks. Int J Comput Assist Radiol Surg. 2023;18(1): 45-54. [59] ZHANG Z, HE T, HUANG L, et al. Immune gene prognostic signature for disease free survival of gastric cancer: translational research of an artificial intelligence survival predictive system. Comput Struct Biotechnol J. 2021;19:2329-2346. [60] SARAJLIC P, PLUNDE O, FRANCO-CERECEDA A, et al. Artificial intelligence models reveal sex-specific gene expression in aortic valve calcification. JACC Basic Transl Sci. 2021;6(5):403-412. [61] HE T, HUANG L, LI J, et al. Potential prognostic immune biomarkers of overall survival in ovarian cancer through comprehensive bioinformatics analysis: a novel artificial intelligence survival prediction system. Front Med (Lausanne). 2021;8:587496. [62] YEO YH, SAMAAN JS, NG WH, et al. Assessing the performance of ChatGPT in answering questions regarding cirrhosis and hepatocellular carcinoma. Clin Mol Hepatol. 2023;29(3):721-732. [63] GRüNEBAUM A, CHERVENAK J, POLLET SL, et al. The exciting potential for ChatGPT in obstetrics and gynecology. Am J Obstet Gynecol. 2023;228(6):696-705. |
| [1] | Xu Zhenhua, Li Yanjie, Qin Hewei, Liu Haoyuan, Zhu Bochao, Wang Yupu. Traditional Chinese medicine monomer in treatment of neuroinflammation after spinal cord injury: effects of nuclear transcription factor kappa B signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 590-598. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||