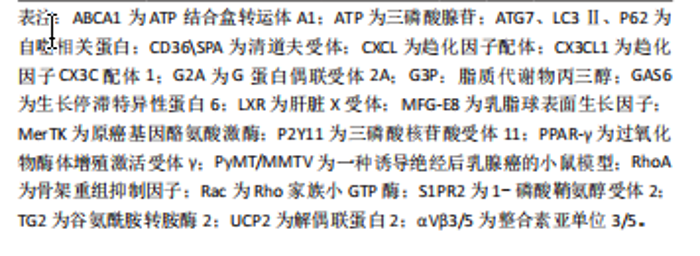
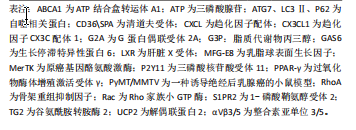
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (2): 430-440.doi: 10.12307/2025.273
Macrophage efferocytosis: a new target for the treatment of obesity-related metabolic diseases #br#
#br#
Yang Fengying1, Zhao Yuqing1, You Huijuan1, Zhang Pengyi1, Chen Yan1, Wang Qinglu1, Liu Yingying2
- 1College of Sports and Health, Shandong Sport University, Jinan 250102, Shandong Province, China; 2Research Center of Aerospace Medicine, Air Force Specialized Medical Center of PLA, Beijing 100142, China
-
Received:2024-01-10Accepted:2024-03-02Online:2025-01-18Published:2024-05-27 -
Contact:Liu Yingying, Research Center of Aerospace Medicine, Air Force Specialized Medical Center of PLA, Beijing 100142, China -
About author:Yang Fengying, MD, College of Sports and Health, Shandong Sport University, Jinan 250102, Shandong Province, China -
Supported by:Shandong Provincial Natural Science Foundation, No. ZR2022MH163 (to YFY)
CLC Number:
Cite this article
Yang Fengying, Zhao Yuqing, You Huijuan, Zhang Pengyi, Chen Yan, Wang Qinglu, Liu Yingying . Macrophage efferocytosis: a new target for the treatment of obesity-related metabolic diseases #br#
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
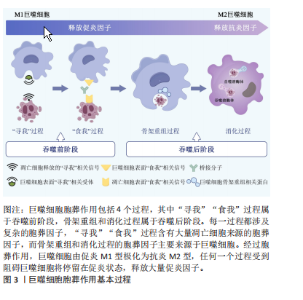
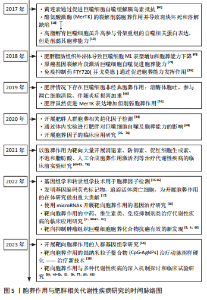
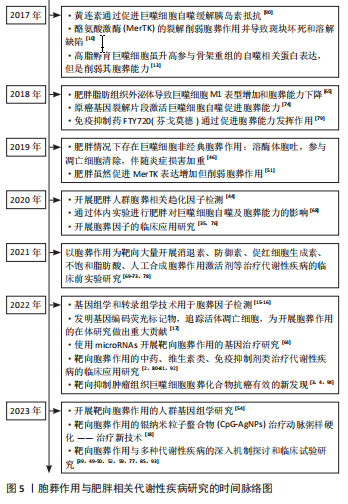
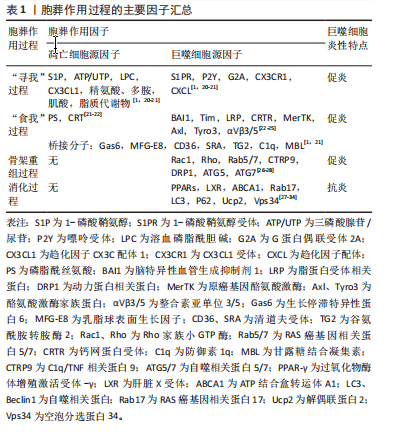
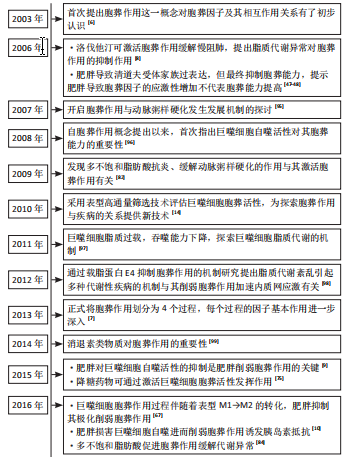
2.1 胞葬作用与肥胖相关代谢性疾病研究的时间脉络 有研究于2003年首次将吞噬细胞清除凋亡细胞的过程定义为胞葬作用,自此胞葬作用引起诸多学者关注,2013年有研究将胞葬作用过程分为4个阶段,每个阶段的胞葬因子及其相互作用关系有了进一步认识[6-7]。在2006年,洛伐他汀通过激活胞葬作用缓解慢阻肺的研究结果引起人们关于脂质代谢紊乱与胞葬作用关系的重视[8]。在2006-2017年,研究主要集中在肥胖对胞葬作用影响机制方面,其中肥胖对巨噬细胞自噬的抑制被认为是肥胖抑制胞葬作用的关键,其他诸多胞葬因子存在肥胖状态下的应激性增强但胞葬能力下降的现象[9-13]。2017年至今,涌现出大量的应用性研究成果,包括对已有药物与胞葬作用关系的深入解读,亦包括靶向胞葬作用的新化合物的研发应用。至今关于胞葬作用与肥胖相关代谢性疾病的研究无论从研究的方法技术还是治疗策略均在不断创新,如2010年采用表型高通量流式细胞筛选技术评估巨噬细胞胞葬能力[14],在2022年采用基因组学和转录组学技术检测胞葬因子[15-16],采用基因编码荧光标记技术追踪活体凋亡细胞和开展靶向胞葬作用的基因治疗[17],以及2023年银纳米螯合技术靶向胞葬作用治疗动脉粥样硬化等[18],为该领域的深入探索提供可能。 2.2 胞葬作用 胞葬作用是吞噬细胞清除凋亡细胞的过程。人体每天有数亿个细胞发生凋亡,凋亡细胞必须被快速高效地清除以免继发性坏死释放细胞毒性物质触发炎性损伤。在参与胞葬作用的专职或非专职吞噬细胞中,巨噬细胞发挥胞葬作用的效果最为明显[1]。经过胞葬作用的巨噬细胞由促炎性M1型转化为抗炎性M2型,并释放转化生长因子β和白细胞介素10等抗炎因子以及促进特异炎症消退介质表达[1,19-20]。因此,通过胞葬作用,一方面清除凋亡细胞,另一方面实现巨噬细胞由促炎型向抗炎型的转化,共同维持机体炎症稳态。 经典的胞葬作用包括“寻我”“食我”、细胞骨架重组和消化4个过程,“寻我”和“食我”过程属于吞噬前阶段,细胞骨架重组和消化过程属于吞噬后阶段,每个过程均涉及复杂的信号分子[21],见图3。"
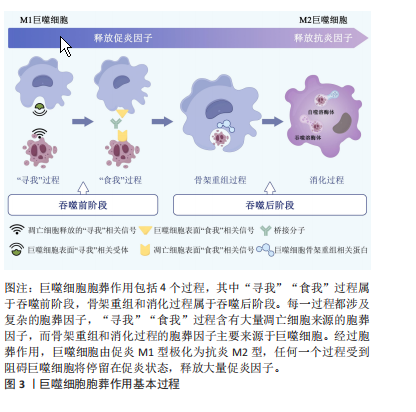
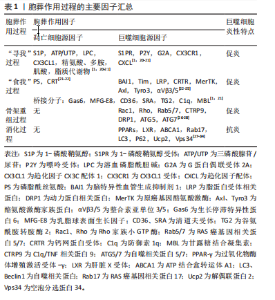
“寻我”阶段是凋亡细胞释放多种因子作为“寻我”信号被巨噬细胞表面相应“寻我”受体感知并产生趋化运动的过程。目前发现的“寻我”信号主要有4对:鞘氨醇-1-磷酸(sphingosine-1-phosphat,S1P)及其受体S1PR,三磷酸核苷酸(triphosphate nucleotides,ATP/UTP)及其受体P2Y,趋化因子CX3C配体1(CX3-C motif chemokine ligand 1,CX3CL1)及其受体CX3CR1,溶血磷脂酰胆碱(lysophosphatidylcholine,LPC)及其受体G2A(G2A family of G-protein-coupled receptors,G2A)[21]。此外,凋亡细胞释放的多胺、脂溶性维生素和脂质代谢物等均可作为寻我信号被巨噬细胞表面的“寻我”受体感知[20]。“食我”阶段是凋亡细胞表面暴露的“食我”信号与巨噬细胞表面“食我”受体的识别和结合的过程。磷脂酰丝氨酸(phosphatidylserine,PS)和钙网蛋白(calreticulin,CRT)是凋亡细胞释放的最主要“食我”信号,可与巨噬细胞表面的同源受体直接结合,也可先与多种桥接分子结合后[22],再与巨噬细胞表面的脑组织特异性血管生成抑制因子(brain-specific angiogenesis inhibitor,BAI)、T细胞免疫球蛋白黏蛋白分子家族(T cell immunoglobulin mucin,TIM)、酪氨酸激酶家族(MerTK、Axl和Tyro3)[23]、整合素3/5(αVβ3/5)、钙网蛋白受体(calreticulin recepter,CRTR)等结合完成“食我”过程[24-25]。 目前研究已发现的桥接分子主要有生长停滞特异性蛋白6(Growth-arrest specific protein 6,Gas6)、乳脂球表面生长因子(milk fat globule epidermal growth factor 8,MFG-E8)、甘露糖结合凝集素( mannose binding lectin,MBL)、转谷氨酰胺酶2 (transglutaminase 2,TG2)、防御素(C1q)及清道夫受体CD36和清道夫受体A(scavenger receptor type A,SRA)等[1,21]。骨架重组是吞噬细胞与凋亡细胞结合后,通过激活Rho家族小GTP酶,如GTPase 1 (Rac1)及Ras家族成员A(Ras homolog family member A,RhoA)等,使细胞骨架凹陷并形成“吞噬杯”(phagocytic cup)以完成内吞的过程[26]。此外,自噬相关蛋白5 (autophagy-related protein 5,ATG5)及ATG7等亦参与了骨架重组[27-28]。巨噬细胞对凋亡细胞的消化需依赖溶酶体实现。除了RAS癌基因相关蛋白RAB17(RAS oncogene family,Rab17)参与的吞噬溶酶体形式[29],还存在微管相关蛋白质轻链3(microtubule-associated proteins light chain 3,LC3)相关的吞噬(LC3-associated phagocytosis,LAP)形式,即自噬溶酶体形式[27-28],其中解偶联蛋白2(uncoupling protein 2,Ucp2)积极参与了LAP消化过程并被认为与凋亡细胞状态关系更紧密[30-31]。胞葬降解产物通过肝脏X受体(liver X receptor,LXR)-ATP结合盒转运体A1(ATP binding cassette transporter A1,ABCA1)通路及时转运,也是决定消化效率的重要环节[32-33]。需要强调的是,胞葬作用各个因子之间相互关联并受过氧化物酶体增殖激活受体γ(peroxisome proliferator-activated receptor-γ,PPAR-γ)信号通路的调控,PPAR-γ敲除的巨噬细胞无法实现胞葬作用,停留在促炎M1型并诱发机体炎症损 伤[34]。 鉴于胞葬作用涉及凋亡细胞和巨噬细胞间的对话,经过对上述胞葬因子的进一步分析,文章将各个阶段的胞葬因子归纳为凋亡细胞源性因子和巨噬细胞源性因子,有助于研究者们更清晰地理解每个阶段凋亡细胞和巨噬细胞对胞葬作用影响的相对主导性,见表1。在吞噬前阶段,大量胞葬因子来源于凋亡细胞,因此这些因子表达的增加仅代表胞葬作用的需求增加,不能作为胞葬活性的判断依据;在吞噬后阶段,巨噬细胞内吞载量和消化降解"
能力直接决定胞葬作用效率,且巨噬细胞完成吞噬后处理任务将伴随M1表型向M2表型的转化及抗炎因子释放,这一特点被认为是判断胞葬作用效率的有效依据。因此,当细胞凋亡增加时,释放大量“寻我”信号,持续募集巨噬细胞,使其围绕在凋亡细胞周围,并释放促炎因子;若巨噬细胞内在功能受到抑制,必然影响胞葬作用效率,导致促炎因子持续释放,再加上凋亡细胞的堆积,可共同威胁内环境的炎症稳态。 2.3 肥胖对巨噬细胞胞葬作用的影响 巨噬细胞是脂肪组织的重要组成部分。在瘦人群体中,巨噬细胞数量占所有脂肪组织细胞数量约10%,且以抗炎效应的M2型常驻巨噬细胞(resident macrophage)为主,而在肥胖者中可达到40%-50%,主要为促炎M1型,且多数是外来浸润性巨噬细胞[35]。肥胖情况下,肥大脂肪细胞凋亡增加,血液中大量骨髓来源的单核细胞在单核细胞趋化因子驱动下浸润入脂肪组织,并分化为巨噬细胞围绕在凋亡肥大脂肪细胞周围形成冠状结构(crown-like structures,CLS),见 图4,此时,巨噬细胞胞葬作用各个过程均被干扰,释放大量促炎因子,引起局部和系统炎症损害[1,36-37]。研究发现肥胖相关代谢性疾病,如动脉粥样硬化、非酒精性脂肪肝、神经退行性疾病以及癌症等的发生和发展均与胞葬作用有关[38-41]。因此,理清肥胖对胞葬作用各个过程的影响机制对于探索脂肪组织炎症反应及其相关的代谢性疾病的防治策略至关重要。 2.3.1 肥胖导致“寻我”信号扩增引起的炎症反应 肥胖状态下,脂肪组织扩张和脂质过氧化应激等效应不仅导致脂肪细胞凋亡增加,还加速其他组织细胞凋亡,由此释放大量“寻我”信号,此时脂肪组织常驻巨噬细胞胞葬作用无法及时清除凋亡脂肪细胞,因此血液中的单核"


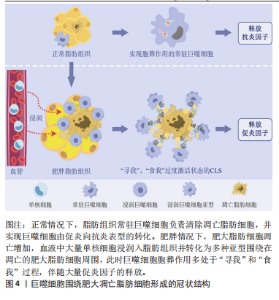
细胞被持续募集入脂肪组织并转化为各种巨噬细胞亚型围绕在坏死脂肪细胞周围形成冠状结构,同时释放大量促炎因子引起局部和系统炎症反应[42]。凋亡细胞释放大量肌酸、ATP、精氨酸、多胺以及丙三醇等脂质代谢物,并诱导巨噬细胞S1PR2和P2Y11表达增高已被近期的基因组学和转录组学研究进一步证实[15,43]。除了上述“寻我”信号激增外,关于肥胖对“寻我”过程影响的直接研究主要集中在巨噬细胞的趋化运动。高糖摄入诱发的肾小管上皮细胞凋亡增加,将诱导巨噬细胞“寻我”受体CX3CL1表达升高,增加其趋化能力[16]。 趋化因子(C-X-C基元)配体9(chemokine (C-X-C motif) ligand 9,CXCL9)、CXCL10和CXCL11也是巨噬细胞表面重要的趋化因子,在“寻我”信号诱导下可驱使巨噬细胞向凋亡细胞趋化,人群研究显示肥胖者血清CXCL9/10/11水平显著高于正常体重人群,但肥胖人群普遍存在的胰岛素抵抗和血清促炎因子增加现象提示巨噬细胞胞葬作用未增强[44]。LUMENG等[45]研究显示肥胖小鼠单核细胞趋化蛋白1(monocyte chemoattractic protein-1,MCP-1/CCL2)的特异受体CC趋化因子受体2(CC chemokine receptor 2,CCR2)表达显著升高,且脂肪组织巨噬细胞表型丰度随着肥胖程度的增加逐渐由M2型向M1型转变,进一步说明胞葬作用减弱。总之,肥胖状态下细胞凋亡增加,释放大量“寻我”信号,持续募集巨噬细胞,同时引起巨噬细胞趋化能力增强和S1PR2和P2Y11表达增加,但并不意味着胞葬作用增强,巨噬细胞的持续大量募集反而成为脂肪组织炎症损害的重要诱因。 2.3.2 “食我”信号过度应激加重炎症损伤 肥胖从多个角度对胞葬作用“食我”过程产生干扰。其中部分“食我”分子在肥胖情况下表达升高,更像是肥胖诱导的应激反应,胞葬作用效率未见提高。MFG-E8是胞葬作用重要的“食我”信号,属于桥接分子,TG2可作为αVβ3的辅助受体稳定其与MFG-E8的链接进而促进胞葬作用[24-25],但高脂环境导致的MFG-E8,αVβ3/5和TG2水平增加并没有增强胞葬作用[12,46],反而导致促炎因子的释放。研究发现TG2敲除(TG2-/-)巨噬细胞清除凋亡脂肪细胞的能力显著优于正常巨噬细胞,但其发挥作用并非通过经典的胞葬作用,而是通过促进αVβ3的表达,进而刺激巨噬细胞溶酶体胞吐效应实现的[46]。上述研究结果说明,高脂环境下MFG-E8,TG2和αVβ3/5的高表达不能促进胞葬作用,反而加重机体炎症状态。与此类似,正常情况下清道夫受体CD36和清道夫受体A(scavenger receptor type A,SRA)可促进胞葬作用,然而高脂环境下,CD36和SRA过表达抑制磷脂酰肌醇3激酶(phosphatidylinositol 3 kinase,PI3K)/蛋白激酶B(Protein kinase B,PKB)信号通路活性,导致胞葬作用受损及巨噬细胞凋亡增加[47-48]。虽然通过激活MertK促进胞葬作用对心血管疾病治疗有效[49-50],但是,高脂膳食干预的研究发现肥胖显著升高小鼠脂肪组织及乳腺组织MerTK的表达同时加重炎症状态[40,51],说明肥胖对MerTK的应激性增强不代表胞葬活性升高;GAS6也是“食我”信号中的桥接分子,能促进“食我”过程,与上述“食我”因子不同的是,GAS6未表现出因高脂环境影响而应激性高表达的特点。肥胖小鼠以及载脂蛋白敲除(Apoe-/-)小鼠GAS6水平均降低,从而抑制滑膜巨噬细胞胞葬作用,导致巨噬细胞停滞在促炎M1状态,进一步加剧骨关节炎程度[41,52]。综合上述研究,肥胖情况下巨噬细胞胞葬作用减弱,同时伴随“食我”因子CD36,SPA,MFG-E8,TG2及MerTK应激性高表达,以及GAS6表达降低,即在肥胖条件下目前发现仅有GAS6表达水平与胞葬作用活性是协同的,提示GAS6可能是靶向胞葬作用干预代谢性疾病更稳定的靶点。然而,“食我”因子众多,揭示肥胖对更多“食我”因子的影响及其与胞葬作用关系还需进一步探讨。 2.3.3 肥胖阻碍巨噬细胞骨架重组抑制胞葬作用 相对于“寻我”“食我”过程,肥胖对骨架重组过程的影响更显著。棕榈酸酯与骨髓源巨噬细胞共育抑制了胞葬作用,但除了CD36升高外,其他“寻我”“食我”因子均未发生显著变化,然而骨架重组抑制因子RhoA表达显著增加,阻碍“吞噬杯”形成[53]。此外,Rac1作为Rho家族小GTP酶,主要通过促进巨噬细胞骨架重组进而促进胞葬作用效率。人群基因组学研究显示肥胖可促进Rac1表达水平[54],细胞干预实验研究亦证明高糖培养可以显著升高INS-1 832/13细胞Rac1的表达[55],但并未发现胞葬作用增强现象[56]。另外,虽然自噬相关蛋白ATG7参与了细胞骨架重组但对小鼠RAW264.7巨噬细胞进行的研究发现虽然棕榈酸酯孵育促进ATG7的表达,但并没有促进巨噬细胞自噬活性及其胞葬作用[13]。骨架重组蛋白表增加但吞噬能力并未增强可能与冠状结构中巨噬细胞溶酶体胞吐作用增强有关[47]。需要强调的是,自噬蛋白不仅参与细胞骨架重组阶段,更主要的是参与胞葬作用的消化阶段[27-28],并且自噬是巨噬细胞降解内吞物的关键途径之一。因此,巨噬细胞自噬活性对胞葬作用效率起关键作用,肥胖对自噬活性的抑制将直接影响胞葬作用效率。 2.3.4 肥胖抑制胞葬作用消化效率引起代谢紊乱 目前,关于肥胖对胞葬作用消化效率影响的研究主要体现在肥胖抑制自噬相关LAP途径。自噬相关LAP途径和吞噬溶酶体途径是目前发现的巨噬细胞消化降解凋亡细胞的两种方式[57-58]。自噬是细胞内清除变性蛋白质、细胞器以及外来微生物等的降解过程,对维持细胞内环境稳态至关重要,也是巨噬细胞发挥胞葬作用不可或缺的重要因素[59]。 自噬对细胞能量状态非常敏感,能量过剩可显著抑制自噬活性[60]。自噬过程中涉及多个自噬相关蛋白的参与,比如ATG7主要参与自噬膜的诱发(initiation),LC3主要参与自噬体的构建,在这一过程中LC3由未酯化的LC3Ⅰ酯化为LC3Ⅱ形式,因此,LC3Ⅱ/LC3Ⅰ比值升高曾被人为是判断自噬激活的标志,但越来越多的研究提示自噬体内包裹物的彻底降解才能代表自噬完成[60]。 细胞内错误折叠的蛋白和功能障碍的细胞器将发生泛素化,p62充当泛素化蛋白与自噬体之间的“桥梁”,靶向自噬体,以便进行降解,在自噬降解过程中必然伴随p62蛋白的降解,因此LC3Ⅱ/LC3Ⅰ比值升高和p62表达下降才是自噬活性的有效评价标准[13,60]。用棕榈酸酯孵育骨髓源巨噬细胞,虽然巨噬细胞LC3Ⅱ/LC3Ⅰ比值升高,但p62表达亦升高的现象说明自噬体形成阶段活跃,或者自噬蛋白参与骨架重组增强,但直接参与自噬降解的p62蛋白未能随着溶酶体降解而下降,提示LAP降解效率下降,自噬通量检测结果也进一步证实棕榈酸酯孵育与正常孵育条件相比骨髓源巨噬细胞胞葬能力显著降低[13,53]。 KRATZ [61]等研究提示PPAR-γ及p62是决定脂肪组织巨噬细胞代谢活性和炎症状态的两个最关键蛋白。此外,在体和离体实验研究均发现高脂环境下Ucp2表达增加,但巨噬细胞自噬效率并未提高,同时胞葬作用被抑制导致促炎因子释放增加[31,62-63]。 MicroRNAs(miRNAs/miRs)是小型、高度保守的非编码RNA分子,在维持胆固醇平衡中起关键作用。肥胖者miRs表达增加,并以抑制巨噬细胞自噬活性的方式抑制胞葬作用,并加速动脉粥样斑块的形成,用miR-33抗体阻断巨噬细胞miR-33后,自噬活性增加,进而抑制动脉粥样硬化斑块坏死核的形成[64]。用高脂膳食诱导的肥胖小鼠内脏脂肪组织外泌体干预RAW264.7巨噬细胞结果抑制ABCA1和LXR的表达同时肿瘤坏死因子α、白细胞介素6、核转录因子κB-p65升高,以及促炎M1型巨噬细胞比例增加[65],进一步证明肥胖对巨噬细胞代谢能力和胞葬作用活性的抑制作用。因此,肥胖条件下虽然自噬蛋白LC3Ⅱ,ATG7及参与自噬的Ucp2均表达增高,但多项研究一致认为P62未能被自噬溶酶体降解的现象说明存在“自噬空泡”现象,实际上自噬效率并未提高,同时巨噬细胞PPAR-γ[66-67]、ABCA1和LXR表达下降及促炎表型丰度增加的证据均表明,肥胖对胞葬作用消化阶段的显著抑制作用。 综上所述,肥胖对多数“寻我”“食我”和细胞骨架重组信号表现出激活效应,但巨噬细胞消化能力的明显降低以及局部和系统严重的炎性损害,表明肥胖最终对胞葬作用的抑制效应,见表2。实际上,肥胖情况下胞 "
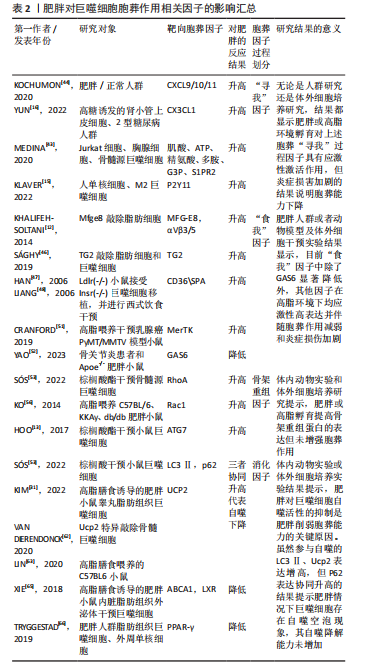
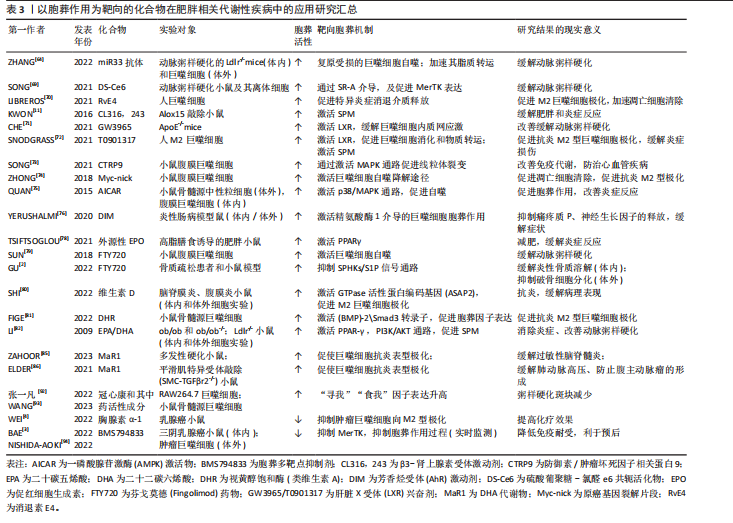
葬作用的障碍以及诱发的炎症状态主要源于冠状结构中的浸润巨噬细胞[24],在肥大凋亡脂肪细胞释放的“寻我”信号诱导下,多个浸润巨噬细胞结合在肥大凋亡脂肪细胞周围,依赖溶酶体胞吐的形式对抗凋亡脂肪细胞,同时释放大量促炎因子导致机体炎症损害,见图4。因此,肥胖状态下“寻我”“食我”和细胞骨架重组信号分子的激活不代表胞葬作用增强,以消化因子的表达水平来反映胞葬作用活性更可靠。鉴于自噬对胞葬作用消化效率的重要性,以巨噬细胞自噬为靶向的深入研究可能是探索防治肥胖相关代谢性疾病的重要切入点,一些以巨噬细胞自噬为靶向的治疗策略[68-69]在动脉粥样硬化等疾病动物模型中疗效显著,进一步说明巨噬细胞自噬活性对胞葬作用的重要性。 2.4 以胞葬作用为靶向的化合物在肥胖相关代谢性疾病中的应用现状 鉴于巨噬细胞胞葬作用在肥胖相关代谢性疾病中的重要性,以胞葬作用为靶向,缓解肥胖介导的炎症反应及代谢性疾病的有效化合物备受关注。消退素类(resolvin E4,RvE4)[70],β3-肾上腺素受体激动剂(CL316,243)[11],人工合成的LXR激动剂GW3965和T0901317均可通过促进巨噬细胞极化和特异炎症消退介质的释放发挥治疗作用[71-72]。防御素/肿瘤坏死因子相关蛋白9(C1q/TNF-related protein 9,CTRP9)可以激活 MAPK信号通路,加速线粒体裂变,促进小鼠腹膜巨噬细胞胞葬作用,防治心血管疾病[73]。体外实验发现原癌基因裂解片段(Myc-nick)可以激活小鼠腹膜巨噬细胞LAP降解途径,促进凋亡细胞清除[74]。阿卡地新核糖苷(Acadesine/AICA riboside,AICAR),可激活巨噬细胞p38/MAPK通路促进胞葬作用治疗胰岛素抵抗[75]。针对上述分子开展的研究均来源于动物实验,其临床应用仍需进一步探讨。 随着基础和转化研究的不断推进,靶向巨噬细胞胞葬作用药物的临床推广运用已经取得了创新性进展。芳香烃受体(AhR)激动剂二吲哚甲烷(3,3’-diindolylmethane,DIM)的应用已处于临床前阶段,表现出良好的抗癌效果[76]。最新研究发现DIM可通过激活AhR/E2相关因子核蛋白(nuclear factor E2-related factor 2,Nrf2)/精氨酸酶1 (arginase-1,Arg-1)通路,促进巨噬细胞胞葬作用缓解肠炎症状[77]。但DIM对癌症治疗有效的机制是否与胞葬作用相关目前未见研究报道。人工合成外源性EPO可以激活巨噬细胞PPARγ信号通路,促进胞葬作用,且已运用到临床治疗多种疾病[78]。FTY720(药物名称:芬戈莫德)是一种免疫抑制剂,临床上用于治疗多种炎症和免疫相关疾病,芬戈莫德属于S1P类似物,可显著促进胞葬作用[2,79]。 维生素D和维生素A类似物可以通过合成“食我”信号分子、促进细胞骨架重组、激活自噬等方式促进胞葬作用[80-81],发挥抗炎、抗病毒及缓解动脉粥样硬化等治疗效果。最新研究显示不饱和脂肪酸[82-84]、及其代谢物maresin 1 (MaR1)可以缓解多发性硬化小鼠的炎症和髓鞘脱失[85],以及有效缓解肺动脉高压和防止腹主动脉瘤的形成[86],其效果均与激活胞葬作用相关。除了EPO、FTY720,其他一些临床常见药物,例如他汀类降脂药、降糖药物、褪黑素以及黄连素缓解胰岛素抵抗治疗效果的发挥也与激活胞葬作用有关[87-90]。王建茹等[91]从Gene Cards数据库中检索与胞葬作用相关的靶点及其匹配的化合物进而通过中药系统药理学数据库与分析平台(traditional chinese medicine systems pharmacology database and analysis platform,TCMSP)筛选与上述靶点相关的中药活性成分,为靶向胞葬作用的中药研制提供思路。后期该团队对临床常用药物冠心康及其有效成分与胞葬作用的关系进行了大量研究,发现其可以升高巨噬细胞胞葬作用进而改善动脉粥样硬化[92-93]。然而,研究过程中发现抑制胞葬作用的化合物,如BMS794833和胸腺素α-1呈现出良好的抗癌效果[3-4,94],提示靶向抑制肿瘤组织巨噬细胞胞葬作用,同时减少对正常组织胞葬作用的干扰,在抗癌药物研发中的重要意义。 总之,胞葬作用涉及复杂的分子和信号通路[95-99],潜在的干预靶点仍需进一步探索。鉴于部分胞葬分子在生理和病理条件下表现出的两面性,以及胞葬某个阶段的活跃与胞葬作用实效之间的矛盾,加之肿瘤等特殊病理组织免疫的特殊性,大量的临床前实验研究和临床应用研究依然任重道远,见表3。"
| [1] TAJBAKHSH A, GHEIBIHAYAT SM, KARAMI N, et al. The regulation of efferocytosis signaling pathways and adipose tissue homeostasis in physiological conditions and obesity: current understanding and treatment options. Obes Rev. 2022;23(10):e13487. [2] GU M, PAN B, CHEN W, et al. SPHK Inhibitors and zoledronic acid suppress osteoclastogenesis and wear particle-induced osteolysis. Front Pharmacol. 2022;12:794429. [3] BAE SH, KIM JH, PARK TH, et al. BMS794833 inhibits macrophage efferocytosis by directly binding to MERTK and inhibiting its activity. Exp Mol Med. 2022;54(9):1450-1460. [4] WEI YT, WANG XR, YAN C, et al. Thymosin α-1 reverses M2 polarization of tumor-associated macrophages during efferocytosis. Cancer Res. 2022;82(10):1991-2002. [5] DE A BOLETI AP, DE O CARDOSO PH, F FRIHLING BE, et al. Adipose tissue, systematic inflammation, and neurodegenerative diseases. Neural Regen Res. 2023;18(1):38-46. [6] DECATHELINEAU AM, HENSON PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem. 2003;39:105-117. [7] HOCHREITER-HUFFORD A, RAVICHANDRAN KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5(1):a008748. [8] MORIMOTO K, JANSSEN WJ, FESSLER MB, et al. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol. 2006;176(12):7657-7665. [9] LIU K, ZHAO E, ILYAS G, et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy. 2015;11(2):271-284. [10] CAI B, THORP EB, DORAN AC, et al. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest. 2017;127(2):564-568. [11] KWON HJ, KIM SN, KIM YA, et al. The contribution of arachidonate 15-lipoxygenase in tissue macrophages to adipose tissue remodeling. Cell Death Dis. 2016;7(6):e2285. [12] KHALIFEH-SOLTANI A, MCKLEROY W, SAKUMA S, et al. Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nat Med. 2014;20(2):175-183. [13] HOO RL, SHU L, CHENG KK, et al. Adipocyte fatty acid binding protein potentiates toxic lipids-induced endoplasmic reticulum stress in macrophages via inhibition of Janus kinase 2-dependent autophagy. Sci Rep. 2017;7:40657. [14] MUSLIN AJ. Phenotypic high-throughput screening in atherosclerosis research: focus on macrophages. J Cardiovasc Transl Res. 2010;3(5): 448-453. [15] KLAVER D, GANDER H, DOBLER G, et al. The P2Y11 receptor of human M2 macrophages activates canonical and IL-1 receptor signaling to translate the extracellular danger signal ATP into anti-inflammatory and pro-angiogenic responses. Cell Mol Life Sci. 2022;79(10):519. [16] YUN J, REN J, LIU Y, et al. MicroRNA (miR)-590-3p alleviates high-glucose induced renal tubular epithelial cell damage by targeting C-X3-C motif chemokine ligand 1 (CX3CL1) in diabetic nephropathy. Bioengineered. 2022;13(1):634-644. [17] RAYMOND MH, DAVIDSON AJ, SHEN Y, et al. Live cell tracking of macrophage efferocytosis during Drosophila embryo development in vivo. Science. 2022;375(6585):1182-1187. [18] TANG C, WANG H, GUO L, et al. CpG-conjugated silver nanoparticles as a multifunctional nanomedicine to promote macrophage efferocytosis and repolarization for atherosclerosis therapy. ACS Appl Mater Interfaces. 2023. doi: 10.1021/acsami.3c11227.
[19] TAJBAKHSH A, REZAEE M, KOVANEN PT, et al. Efferocytosis in atherosclerotic lesions: Malfunctioning regulatory pathways and control mechanisms. Pharmacol Ther. 2018;188:12-25. [20] TAJBAKHSH A, YOUSEFI F, ABEDI SM, et al. The cross-talk between soluble “Find me” and “Keep out” signals as an initial step in regulating efferocytosis. J Cell Physiol. 2022;237(8):3113-3126. [21] 金妍君,廖善婷,王岩.胞葬作用与炎症相关性疾病的研究进展[J].徐州医科大学学报,2023,43(3):230-234. [22] BARCZYK M, CARRACEDO S, GULLBERG D. Integrins. Cell Tissue Res. 2010;339(1):269-280. [23] AEHNLICH P, POWELL RM, PEETERS MJW, et al. TAM receptor inhibition-implications for cancer and the immune system. Cancers (Basel). 2021;13(6):1195. [24] DORAN AC, YURDAGUL A, TABAS I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20(4):254-267. [25] BOADA-ROMERO E, MARTINEZ J, HECKMANN BL, et al. Mechanisms and physiology of the clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol. 2020;21(7):398-414. [26] KIM SY, KIM S, BAE DJ, et al. Coordinated balance of Rac1 and RhoA plays key roles in determining phagocytic appetite. PLoS One. 2017; 12(4):e0174603. [27] ASARE PF, ROSCIOLI E, HURTADO PR, et al. LC3-associated phagocytosis (LAP): a potentially influential mediator of efferocytosis-related tumor progression and aggressiveness. Front Oncol. 2020;10:1298. [28] TANZER MC. You are what you eat and how you digest it! A discussion on inflammatory efferocytosis. Front Cell Dev Biol. 2023;11:1132696. [29] YIN C, ARGINTARU D, HEIT B. Rab17 mediates intermixing of phagocytosed apoptotic cells with recycling endosomes. Small GTPases. 2019;10(3):218-226. [30] PAN P, ZHANG H, SU L, et al. Melatonin balance the autophagy and apoptosis by regulating UCP2 in the LPS-induced cardiomyopathy. Molecules. 2018;23(3):675. [31] KIM DH, KIM HJ, SEONG JK. UCP2 KO mice exhibit ameliorated obesity and inflammation induced by high-fat diet feeding. BMB Rep. 2022; 55(10):500-505. [32] FAULDS MH, ZHAO C, DAHLMAN-WRIGHT K. Molecular biology and functional genomics of liver X receptors (LXR) in relationship to metabolic diseases. Curr Opin Pharmacol. 2010;10(6):692-697. [33] MATSUO M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J Pharmacol Sci. 2022;148(2):197-203. [34] MUKUNDAN L, ODEGAARD JI, MOREL CR, et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15(11):1266-1272. [35] ZATTERALE F, LONGO M, NADERI J, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2020;10:1607. [36] LI X, REN Y, CHANG K, et al. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front Immunol. 2023;14: 1153915. [37] LAZAROV T, JUAREZ-CARREÑO S, COX N, et al. Physiology and diseases of tissue-resident macrophages. Nature. 2023;618(7966):698-707. [38] XIE Y, CHEN H, QU P, et al. Novel insight on the role of Macrophages in atherosclerosis: focus on polarization, apoptosis and efferocytosis. Int Immunopharmacol. 2022;113(Pt A):109260. [39] WANG X, HE Q, ZHOU C, et al. Prolonged hypernutrition impairs TREM2-dependent efferocytosis to license chronic liver inflammation and NASH development. Immunity. 2023;56(1):58-77.e11. [40] TAJBAKHSH A, READ M, BARRETO GE, et al. Apoptotic neurons and amyloid-beta clearance by phagocytosis in Alzheimer’s disease: pathological mechanisms and therapeutic outlooks. Eur J Pharmacol. 2021;895:173873. [41] 俞数,陶翊桀,钱程.胞葬作用对肿瘤发生发展影响的研究进展[J].中国肿瘤生物治疗杂志,2023,30(10):914-918. [42] RÖSZER T. Adipose tissue immunometabolism and apoptotic cell clearance. Cells. 2021;10(9):2288. [43] MEDINA CB, MEHROTRA P, ARANDJELOVIC S, et al. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020; 580(7801):130-135. [44] KOCHUMON S, MADHOUN AA, AL-RASHED F, et al. Adipose tissue gene expression of CXCL10 and CXCL11 modulates inflammatory markers in obesity: implications for metabolic inflammation and insulin resistance. Ther Adv Endocrinol Metab. 2020;11:2042018820930902. [45] LUMENG CN, BODZIN JL, SALTIEL AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007; 117(1):175-184. [46] SÁGHY T, KÖRÖSKÉNYI K, HEGEDŰS K, et al. Loss of transglutaminase 2 sensitizes for diet-induced obesity-related inflammation and insulin resistance due to enhanced macrophage c-Src signaling. Cell Death Dis. 2019;10(6):439. [47] HAN S, LIANG CP, DEVRIES-SEIMON T, et al. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necroticcore formation in advanced atherosclerotic lesions. Cell Metab. 2006;3(4):257-266. [48] LIANG SH, ZHANG W, MCGRATH BC, et al. PERK (eIF2alpha kinase) is required to activate the stress-activated MAPKs and induce the expression of immediate-early genes upon disruption of ER calcium homoeostasis. Biochem J. 2006;393(Pt 1):201-209. [49] GAO J, HUANG C, KONG L, et al. SIRT3 regulates clearance of apoptotic cardiomyocytes by deacetylating frataxin. Circ Res. 2023;133(7):631-647. [50] WANG Y, LIU XY, WANG Y, et al. NOX2 inhibition stabilizes vulnerable plaques by enhancing macrophage efferocytosis via MertK/PI3K/AKT pathway. Redox Biol. 2023;64:102763. [51] CRANFORD TL, VELÁZQUEZ KT, ENOS RT, et al. Effects of high fat diet-induced obesity on mammary tumorigenesis in the PyMT/MMTV murine model. Cancer Biol Ther. 2019;20(4):487-496. [52] YAO Z, QI W, ZHANG H, et al. Down-regulated GAS6 impairs synovial macrophage efferocytosis and promotes obesity-associated osteoarthritis. Elife. 2023;12:e83069. [53] SÓS L, GARABUCZI É, SÁGHY T, et al. Palmitate inhibits mouse macrophage efferocytosis by activating an mTORC1-regulated Rho kinase 1 pathway: therapeutic implications for the treatment of obesity. Cells. 2022;11(21):3502. [54] D’SOUZA SE, KHAN K, JALAL K, et al. The gene network correlation analysis of obesity to type 1 diabetes and cardiovascular disorders: an interactome-based bioinformatics approach. Mol Biotechnol. 2023. doi: 10.1007/s12033-023-00845-5. [55] GAMAGE S, HALI M, CHEN F, et al. CARD9 mediates pancreatic islet beta-cell dysfunction under the duress of hyperglycemic stress. Cell Physiol Biochem. 2022;56(2):120-137. [56] KO SH, LEE JK, LEE HJ, et al. 8-Oxo-2’-deoxyguanosine ameliorates features of metabolic syndrome in obese mice. Biochem Biophys Res Commun. 2014;443(2):610-616. [57] TEPLOVA I, LOZY F, PRICE S, et al. ATG proteins mediate efferocytosis and suppress inflammation in mammary involution. Autophagy. 2013; 9(4):459-475. [58] HECKMANN BL, BOADA-ROMERO E, CUNHA LD, et al. LC3-associated phagocytosis and inflflammation. J. Mol. Biol. 2017;429(23):3561-3576. [59] VARGAS JNS, HAMASAKI M, KAWABATA T, et al. The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol. 2023;24(3):167-185. [60] ESCOBAR KA, COLE NH, MERMIER CM, et al. Autophagy and aging: Maintaining the proteome through exercise and caloric restriction. Aging Cell. 2019;18(1):e12876. [61] KRATZ M, COATS BR, HISERT KB, et al. Metabolic dysfunction drivesa mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20(4):614-625.
[62] VAN DIERENDONCK XAMH, SANCERNI T, ALVES-GUERRA MC, et al. The role of uncoupling protein 2 in macrophages and its impact on obesity-induced adipose tissue inflammation and insulin resistance. J Biol Chem. 2020;295(51):17535-17548.
[63] LIN HY, WENG SW, SHEN FC, et al. Abrogation of toll-like receptor 4 mitigates obesity-induced oxidative stress, proinflammation, and insulin resistance through metabolic reprogramming of mitochondria in adipose tissue. Antioxid Redox Signal. 2020;33(2):66-86. [64] ZHANG X, ROTLLAN N, CANFRÁN-DUQUE A, et al. Targeted suppression of miRNA-33 using pHLIP improves atherosclerosis regression. Circ Res. 2022;131(1):77-90. [65] XIE Z, WANG X, LIU X, et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J Am Heart Assoc. 2018;7(5):e007442. [66] TRYGGESTAD JB, TEAGUE AM, SPARLING DP, et al. Macrophage-derived microRNA-155 increases in obesity and influences adipocyte metabolism by targeting peroxisome proliferator-activated receptor gamma. Obesity (Silver Spring). 2019;27(11):1856-1864. [67] ZHANG M, ZHOU Z, WANG J, et al. MiR-130b promotes obesity associated adipose tissue inflammation and insulin resistance in diabetes mice through alleviating M2 macrophage polarization via repression of PPAR-γ. Immunol Lett. 2016;180:1-8. [68] WANG Z, SEQUEIRA RC, ZABALAWI M, et al. Myeloid atg5 deletion impairs n-3 PUFA-mediated atheroprotection. Atherosclerosis. 2020; 295:8-17. [69] SONG JW, AHN JW, LEE MW, et al. Targeted theranostic photoactivation on atherosclerosis. J Nanobiotechnology. 2021;19(1):338. [70] LIBREROS S, SHAY AE, NSHIMIYIMANA R, et al. A new e-series resolvin: RvE4 stereochemistry and function in efferocytosis of inflammation-resolution. Front Immunol. 2021;11:631319. [71] CHE X, XIAO Q, SONG W, et al. Protective functions of liver X receptor α in established vulnerable plaques: involvement of regulating endoplasmic reticulum-mediated macrophage apoptosis and efferocytosis. J Am Heart Assoc. 2021;10(10):e018455. [72] SNODGRASS RG, BENATZY Y, SCHMID T, et al. Efferocytosis potentiates the expression of arachidonate 15-lipoxygenase (ALOX15) in alternatively activated human macrophages through LXR activation. Cell Death Differ. 2021;28(4):1301-1316. [73] SONG CX, CHEN JY, LI N, et al. CTRP9 enhances efferocytosis in macrophages via MAPK/Drp1-mediated mitochondrial fission and AdipoR1-induced immunometabolism. J Inflamm Res. 2021;14:1007-1017. [74] ZHONG X, LEE H-N, KIM SH, et al. Myc-nick promotes efferocytosis through M2 macrophage polarization during resolution of inflammation. FASEB j. 2018;32(10):5312-5325. [75] QUAN H, KIM JM, LEE HJ, et al. AICAR Enhances the phagocytic ability of macrophages towards apoptotic cells through p38 mitogen activated protein kinase activation independent of AMP-activated protein kinase. PLoS One. 2015;10:e0127885. [76] YERUSHALMI R, BARGIL S, BER Y, et al. 3,3-Diindolylmethane (DIM): a nutritional intervention and its impact on breast density in healthy BRCA carriers. A prospective clinical trial. Carcinogenesis. 2020;41(10): 1395-1401. [77] YANG L, ZHENG C, XIA YF, et al. 3,3’-diindolylmethane enhances macrophage efferocytosis and subsequently relieves visceral pain via the AhR/Nrf2/Arg-1-mediated arginine metabolism pathway. Phytomedicine. 2023;116:154874. [78] TSIFTSOGLOU AS. Erythropoietin (EPO) as a key regulator of erythropoiesis, bone remodeling and endothelial transdifferentiation of multipotent mesenchymal stem cells (MSCs): implications in regenerative medicine. Cells. 2021;10(8):2140. [79] SUN RZ, FAN Y, LIANG X, et al. Rapamycin and FTY720 alleviate atherosclerosis by cross talk of macrophage polarization and autophagy. Biomed Res Int. 2018;2018:1010248. [80] SHI H, DUAN J, WANG J, et al. 1,25(OH)2D3 promotes macrophage efferocytosis partly by upregulating ASAP2 transcription via the VDR-bound enhancer region and ASAP2 may affect antiviral immunity. Nutrients. 2022;14(22):4935. [81] FIGE É, SARANG Z, SÓS L, et al. Retinoids promote mouse bone marrow-derived macrophage differentiation and efferocytosis via upregulating bone morphogenetic protein-2 and Smad3. Cells. 2022;11(18):2928. [82] LI S, SUN Y, LIANG CP, et al. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ Res. 2009;105(11):1072-1082. [83] KWON Y. Immuno-resolving ability of resolvins, protectins, and maresins derived from Omega-3 fatty acids in metabolic syndrome. Mol Nutr Food Res. 2020;64(4):e1900824. [84] LÓPEZ-VICARIO C, RIUS B, ALCARAZ-QUILES J, et al. Pro-resolving mediators produced from EPA and DHA: Overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur J Pharmacol. 2016;785:133-143. [85] ZAHOOR I, NEMATULLAH M, AHMED ME, et al. Pro-resolution lipid mediator maresin-1 ameliorates inflammation, promotes neuroprotection, and prevents disease progression in experimental models of multiple sclerosis. Bio Rxiv. 2023;26:559216. [86] ELDER CT, FILIBERTO AC, SU G, et al. Maresin 1 activates LGR6 signaling to inhibit smooth muscle cell activation and attenuate murine abdominal aortic aneurysm formation. FASEB J. 2021;35(8):e21780. [87] TAJBAKHSH A, GHEIBIHAYAT SM, ASKARI H, et al. Statin-regulated phagocytosis and efferocytosis in physiological and pathological conditions. Pharmacol Ther. 2022;238:108282. [88] AN Y, ZHANG H, WANG C, et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019;33(11):12515-12527. [89] PIVONELLO C, NEGRI M, PATALANO R, et al. The role of melatonin in the molecular mechanisms underlying metaflammation and infections in obesity: a narrative review. Obes Rev. 2022;23(3):e13390. [90] ZHOU H, FENG L, XU F, et al. Berberine inhibits palmitate-induced NLRP3 inflammasome activation by triggering autophagy in macrophages: a new mechanism linking berberine to insulin resistance improvement. Biomed Pharmacother. 2017;89:864-874. [91] 王建茹,李彬,王新陆,等.中药调控胞葬作用物质基础及用药规律的研究[J].中国中医基础医学杂志,2021,27(10):1649-1656. [92] 张一凡,刘萍.冠心康冻干粉通过SENP1/STAT3增强小鼠骨髓源巨噬细胞胞葬作用[J].环球中医药,2022,15(10):1755-1760. [93] WANG J, ZHANG Y, FENG X, et al. Tanshinone IIA alleviates atherosclerosis in LDLR-/-mice by regulating efferocytosis of macrophages. Front Pharmacol. 2023;14:1233709. [94] NISHIDA-AOKI N, GUJRAL TS. Polypharmacologic reprogramming of tumor-associated macrophages toward an inflammatory phenotype. Cancer Res. 2022;82(3):433-446. [95] TABAS I. Apoptosis and efferocytosis in mouse models of atherosclerosis. Curr Drug Targets. 2007;8(12):1288-1296. [96] BIRMINGHAM CL, HIGGINS DE, BRUMELL JH. Avoiding death by autophagy: interactions of Listeria monocytogenes with the macrophage autophagy system. Autophagy. 2008;4(3):368-371. [97] FRISDAL E, LESNIK P, OLIVIER M, et al. Interleukin-6 protects human macrophages from cellular cholesterol accumulation and attenuates the proinflammatory response. J Biol Chem. 2011;286(35):30926-30936. [98] CASH JG, KUHEL DG, BASFORD JE, et al. Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J Biol Chem. 2012;287(33):27876-27884. [99] MCCAULEY LK, DALLI J, KOH AJ, et al. Cutting edge: parathyroid hormone facilitates macrophage efferocytosis in bone marrow via proresolving mediators resolvin D1 and resolvin D2. J Immunol. 2014; 193(1):26-29. |
| [1] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [2] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| [3] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [4] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [5] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [6] | Chang Jinxia, Liu Yufei, Niu Shaohui, Wang Chang, Cao Jianchun. Visualization analysis of macrophage polarization in tissue repair process [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1486-1496. |
| [7] | Chen Yuning, Jiang Ying, Liao Xiangyu, Chen Qiongjun, Xiong Liang, Liu Yue, Liu Tong. Buqi Huoxue Compounds intervene with the expression of related factors and autophagy related proteins in a rat model of cerebral ischemia/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1152-1158. |
| [8] | Liu Lingyun, He Guixin, Qin Weibin, Song Hui, Zhang Liwen, Tang Weizhi, Yang Feifei, Zhu Ziyi, Ou Yangbin . Improvement of myocardial injury by traditional Chinese medicine: mitochondrial calcium homeostasis mediates macrophage autophagy and pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1276-1284. |
| [9] | Bai Jing, Zhang Xue, Ren Yan, Li Yuehui, Tian Xiaoyu. Effect of lncRNA-TNFRSF13C on hypoxia-inducible factor 1alpha in periodontal cells by modulation of #br# miR-1246 #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 928-935. |
| [10] | Zhi Fang, Zhu Manhua, Xiong Wei, Lin Xingzhen. Analgesic effect of acupuncture in a rat model of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 936-941. |
| [11] | Wang Yuru, Li Siyuan, Xu Ye, Zhang Yumeng, Liu Yang, Hao Huiqin. Effects of wogonin on joint inflammation in collagen-induced arthritis rats via the endoplasmic reticulum stress pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1026-1035. |
| [12] | Wang Sifan, He Huiyu, Yang Quan, Han Xiangzhen. miRNA-378a overexpression of macrophage cell line composite collagen sponge: anti-inflammation and tissue repair promotion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 789-799. |
| [13] | Zheng Yitong, Wang Yongxin, Liu Wen, Amujite, Qin Hu. Action mechanism of intrathecal transplantation of human umbilical cord mesenchymal stem cell-derived exosomes for repair of spinal cord injury under neuroendoscopy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7743-7751. |
| [14] | Yu Hui, Yang Yang, Wei Ting, Li Wenli, Luo Wenqian, Liu Bin. Gadd45b alleviates white matter damage in chronic ischemic rats by modulating astrocyte phenotype [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7797-7803. |
| [15] |
Li Zhipeng, Xing Rongxin, Hu Lianghong.
Roles of SOX5 in bone metabolism and prevention of bone diseases and the relationship with exercise#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7589-7600.
|
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||