Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (26): 5501-5510.doi: 10.12307/2025.738
Single-cell sequencing reveals heterogeneity of B cells in osteoporosis patients and their interactions with osteoblasts
Tang Zhi1, Shao Yang2, Li Shaoshuo2, Qi Shubin3, Lu Hengyang4, Wu Mao2, Yang Junfeng2, Wang Jianwei2
- 1Nanjing University of Chinese Medicine, Nanjing 210023, Jiangsu Province, China; 2Wuxi Traditional Chinese Medicine Hospital, Wuxi 214071, Jiangsu Province, China; 3Qingdao Traditional Chinese Medicine Hospital, Qingdao 266500, Shandong Province, China; 4School of Artificial Intelligence and Computer Science, Jiangnan University, Wuxi 214112, Jiangsu Province, China
-
Received:2024-07-24Accepted:2024-09-14Online:2025-09-18Published:2025-02-20 -
Contact:Wang Jianwei, MD, Professor, Doctoral supervisor, Wuxi Traditional Chinese Medicine Hospital, Wuxi 214071, Jiangsu Province, China -
About author:Tang Zhi, Master’s candidate, Nanjing University of Chinese Medicine, Nanjing 210023, Jiangsu Province, China -
Supported by:the National Natural Science Foundation of China, No. 82274546 (to WJW); Wuxi “Double Hundred” Young and Middle-aged Talents in Healthcare, Nos. HB2023072 (to SY) and HB2023074 (to LSS); Jiangsu Provincial Science and Technology Development Program of Traditional Chinese Medicine for Young Talents, No. QN202322 (to LSS); Wuxi Municipal Commission of Healthcare Youth Research Project, No. Q202232 (to LSS)
CLC Number:
Cite this article
Tang Zhi, Shao Yang, Li Shaoshuo, Qi Shubin, Lu Hengyang, Wu Mao, Yang Junfeng, Wang Jianwei. Single-cell sequencing reveals heterogeneity of B cells in osteoporosis patients and their interactions with osteoblasts[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5501-5510.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

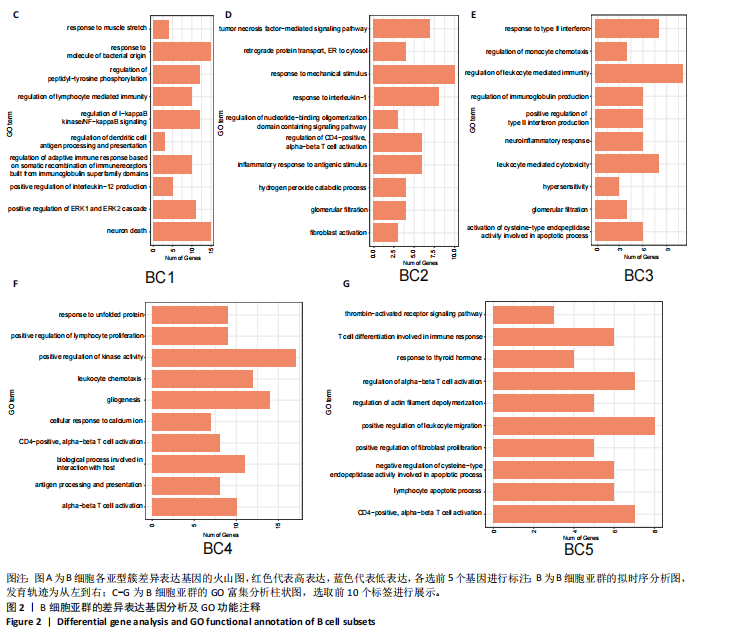
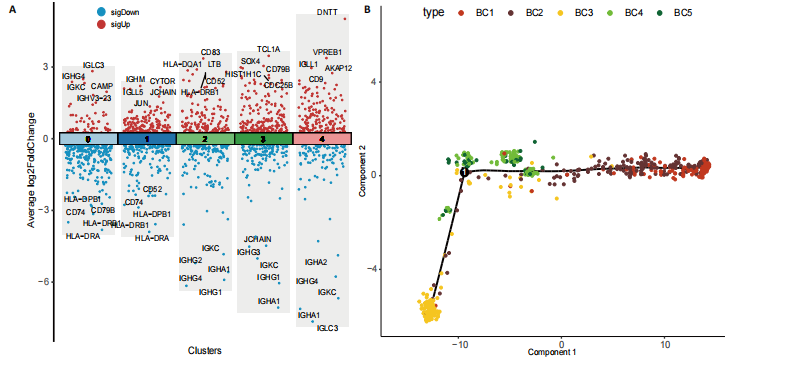
2.1 骨组织细胞及B细胞亚群聚类分析 此次研究使用的人股骨头组织单细胞测序数据来源于GEO数据库GSE 169396和GSE 147287,选取了其中2例骨质疏松症患者和1例非骨质疏松症患者的股骨头测序数据,对样本进行质量控制,使用FindIntegrationAnchors函数和IntegrateData函数进行数据去批次效应整合后得到了23 195个细胞的转录组数据,对这些数据进行降维处理并聚类分群,相关结果见图1。 将各簇的前5个差异表达基因用热图展现出来,见图1E,借助热图标示的差异基因以及众所周知的标记基因识别了10个不同细胞类型,包括破骨细胞、髓样细胞、T细胞、成骨细胞、巨噬细胞、单核细胞、红细胞、B细胞、骨髓间充质干细胞、肥大细胞,见图1A。计算各细胞群在所有细胞中的比例并通过柱状图表示出来,可见骨质疏松症组股骨头组织中破骨细胞比例增加、成骨细胞比例减少,这与骨质疏松症的病理机制及国内外研究相符[12],同时还观察到明显的T细胞比例增加及B细胞比例减少,见图1B,提示免疫细胞在骨质疏松症发病机制中可能发挥了重要作用,促使人们进一步探究。 为了探究B细胞在骨质疏松症中的作用和变化,将上一步识别为B细胞的聚类单独提取出来进一步分析,用同样的方法进行降维处理并通过t-SNE进行可视化,B细胞被分成了5个亚群,见图1C。通过细胞比例柱状图观察到,在B细胞亚群中,与非骨质疏松患者相比,骨质疏松症患者第1,3类群B细胞(BC1、BC3)比例较高,第2类群的B细胞(BC2)比例减少,其余类群B细胞比例变化不显著,见图1D。 2.2 B细胞亚群鉴定及各细胞簇表达特点 对B细胞亚群进行了进一步分析,通过计算各亚型细胞簇标记基因的log后差异倍数和-log10(P值),并通过火山图展示出来,表现基因表达变化的显著性和大小。其中,BC1显著高表达免疫球蛋白重常数γ-4、免疫球蛋白λ常数3等基因;BC2显著高表达免疫球蛋白重常数μ、免疫球蛋白Lambda样多肽5等基因;BC3高表达人Ⅱ类主要组织相容性复合体DRβ1、CD83等基因;BC4高表达T细胞白血病/淋巴瘤1A、CD79B等基因;BC5高表达末端脱氧核苷酸转移酶、前B淋巴细胞基因1等基因,见图2A。BC1高表达的免疫球蛋白λ常数3、免疫球蛋白重常数γ-4、免疫球蛋白K、环磷酸腺苷基因是成熟、衰老效应B细胞的标记基因,BC3高表达的人Ⅱ类主要组织相容性复合体DRβ1、CD83基因是初始B细胞的标记基因[13],这两类细胞在骨质疏松症组股骨组织中的比例较非骨质疏松症组股骨组织明显升高,见图1B。随后对B细胞亚群进行了拟时序分析,发现BC3(黄色)位于发育轨迹的初始段,BC1(红色)位于发育轨迹的末尾段,其余亚型散在分布在中间段,这与鉴定的B细胞亚型相符,见图2B。 对各簇的标记基因进行了GO功能富集,选取了富集显著的前10个功能标签用柱状图展示,见图2C-G。显示BC1富集显著的标签有适应性免疫反应调节、免疫受体的体细胞重组、调节淋巴细胞介导的免疫等,见图2C;BC3富集显著的标签有免疫球蛋白产生的调节、对Ⅱ型干扰"

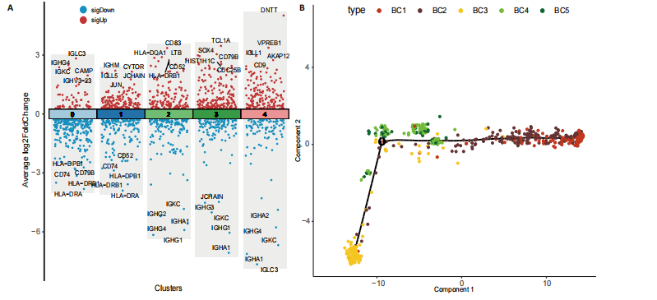
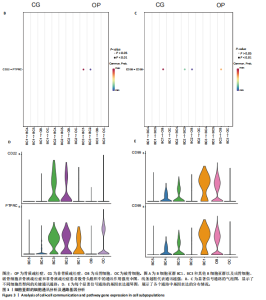
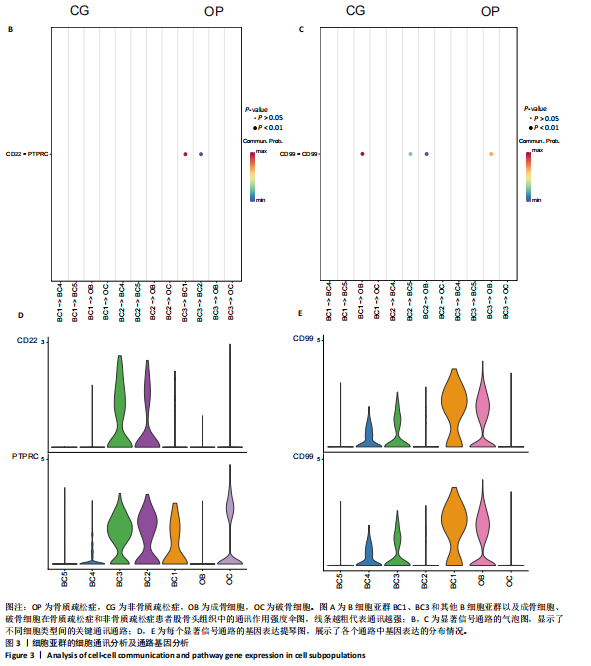
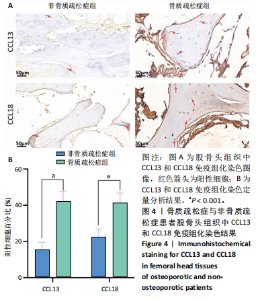
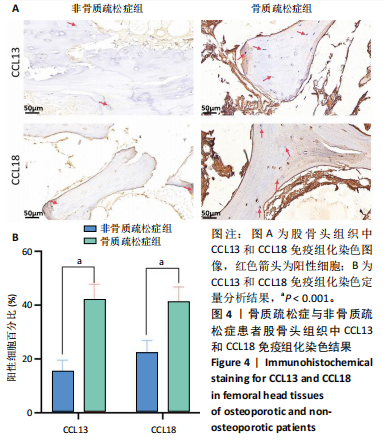
素的应答、半胱氨酸内肽酶参与的凋亡过程、细胞毒性等,见图2E,这些标签进一步验证了鉴定的BC1、BC3细胞亚群类型,充分揭示了它们发挥的功能。 2.3 细胞互作分析及关键通路基因分析 使用CellChat工具对单细胞RNA测序数据进行了细胞间通讯分析,将信号作用强度用伞图可视化。结果显示,与非骨质疏松症组相比,骨质疏松症组股骨头组织中B细胞亚型BC1与成骨细胞间的通讯强度提高,同时BC3与BC1之间的细胞通讯强度也有所提高,见图3A。为了更深入了解细胞间相互作用的通路,通过可视化分析生成了显著信号通路的气泡图,揭示了不同细胞类型间的关键通讯通路,其中BC3与BC1之间的通讯主要富集在CD22-受体型酪氨酸蛋白磷酸酶C信号通路,见图3B;BC1与成骨细胞之间的通讯主要富集在CD99-CD99信号通路,并且BC3与成骨细胞之间的通讯在该信号通路中也有较高的富集,见图3C。 基因表达提琴图有助于人们理解关键信号通路基因在不同细胞类型中的表达分布,为每个显著的信号通路生成了基因表达提琴图,发现CD22和受体型酪氨酸蛋白磷酸酶C基因在BC3、BC2、BC1显著表达,见图3D,而CD99基因在BC1和成骨细胞中显著表达,这些基因在其他类群中表达均不显著,见图3E,这进一步验证了CD22-受体型酪氨酸蛋白磷酸酶C、CD99-CD99信号通路在骨质疏松症患者股骨头组织中的关键作用,以及相关基因CD22、受体型酪氨酸蛋白磷酸酶C、CD99的特征性表达对信号通路和细胞功能的显著影响,揭示了这些通路和基因在免疫调控骨质疏松中的重要作用。 2.4 验证实验结果 2.4.1 免疫组化染色结果 在验证实验中关注免疫相关基因在骨质疏松症患者股骨头组织中的表达和分布,以证明免疫在骨质疏松症中发挥的重要作用。免疫组化是检测组织切片中目标蛋白定位与表达水平的有效工具[14-16],此次实验选择免疫组化检测CCL13和CCL18的表达,因为这些趋化因子在组织中的表达模式和分布对于理解免疫在骨质疏松症病理过程中的作用至关重要。 免疫组化染色分析结果显示,骨质疏松症组股骨头组织中CCL13和CCL18阳性细胞数量明显高于非骨质疏松症组,见图4。说明与非骨质疏松症患者相比,骨质疏松症患者股骨头骨组织中CCL13和CCL18蛋白表达量高。 2.4.2 qPCR检测结果 qPCR是检测特定基因表达水平的高灵敏度方法[17-19]。为验证人Ⅱ类主要组织相容性复合体DRβ1、CD83和免疫球蛋白重常数γ-4、免疫球蛋白λ常数3基因在骨质疏松症患者股骨头组织中表达增加,选择qPCR检测上述的基因表达,这些基因在特定免疫细胞中的表达水平可能与B细胞亚群的免疫反应和骨质疏松症机制密切相关。"
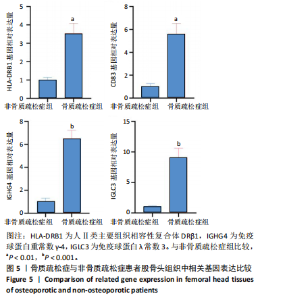
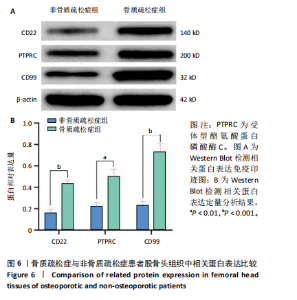
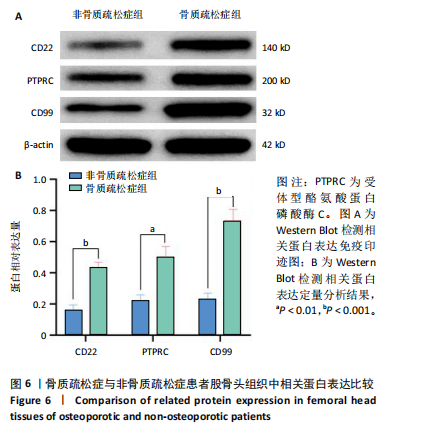
qPCR检测结果显示,骨质疏松症组股骨头组织中人Ⅱ类主要组织相容性复合体DRβ1、CD83和免疫球蛋白重常数γ-4、免疫球蛋白λ常数3 mRNA表达明显高于非骨质疏松症组,见图5。而上述基因在B细胞亚群中均特异性表达,见图2A,进一步验证了BC1、BC3在骨质疏松症后的比例增加。 2.4.3 Western Blot检测结果 Western Blot检测能够提供蛋白质表达水平及其分子质量的信息,能够检测信号传导途径中的关键调节蛋白,提供有关蛋白在细胞信号传导中的功能性线索[20-22]。为了检验骨质疏松症患者CD22-受体型酪氨酸蛋白磷酸酶C和CD99信号通路的表达是否增强,对信号通路相关蛋白进行了Western Blot验证,结果显示骨质疏松症患者股骨头组织中信号通路相关蛋白条带明显增粗,见图6A,定量分析也显示骨质疏松症患者股骨头组织中CD22、受体型酪氨酸蛋白磷酸酶C和CD99蛋白表达明显增高,见图6B,进一步证明了上述信号通路的活跃,在骨质疏松症中发挥着重要作用。"

| [1] 张玉,游如旭,张聪,等.骨质疏松症治疗药物合理应用专家共识(2023)[J].中国医院药学杂志,2024,44(9):985-1006. [2] TRÉMOLLIÈRES F. [Menopause hormone therapy and osteoporosis]. Rev Prat. 2020;70(10):1097-1099. [3] ZHANG W, GAO R, RONG X, et al. Immunoporosis: Role of immune system in the pathophysiology of different types of osteoporosis. Front Endocrinol (Lausanne). 2022;13:965258. [4] YATSONSKY II D, PAN K, SHENDGE VB, et al. Linkage of microbiota and osteoporosis: A mini literature review. World J Orthop. 2019;10(3): 123-127. [5] 孙成涛,孙广江,戚晓楠,等.单细胞转录组测序技术在骨质疏松症研究中的应用与进展[J].中国组织工程研究,2025,29(13):2812-2821. [6] SAXENA Y, ROUTH S, MUKHOPADHAYA A. Immunoporosis: role of innate immune cells in osteoporosis. Front Immunol. 2021;12:687037. [7] 陈天宁,杨铁毅,邵进,等.免疫相关基因在绝经后骨质疏松症患者外周血白细胞中的表达[J].中国组织工程研究,2020,24(25):4033-4038. [8] SLOVIN S, CARISSIMO A, PANARIELLO F, et al. Single-Cell RNA Sequencing Analysis: A Step-by-Step Overview. Methods Mol Biol (Clifton, NJ). 2021;2284:343-365. [9] LEI Y, TANG R, XU J, et al. Applications of single-cell sequencing in cancer research: progress and perspectives. J Hematol Oncol. 2021;14(1):91. [10] 许业楠,张贤,刘小峰,等.单细胞测序技术在骨质疏松诊疗中的研究进展[J].中国骨质疏松杂志,2024,30(3):463-468. [11] 中华医学会骨质疏松和骨矿盐疾病分会,章振林.原发性骨质疏松症诊疗指南(2022)[J].中国全科医学,2023,26(14):1671-1691. [12] WU S, OHBA S, MATSUSHITA Y. Single-Cell RNA-Sequencing Reveals the Skeletal Cellular Dynamics in Bone Repair and Osteoporosis. Int J Mol Sci. 2023;24(12):9814. [13] CHEN J, TAN Y, SUN F, et al. Single-cell transcriptome and antigen-immunoglobin analysis reveals the diversity of B cells in non-small cell lung cancer. Genome Biol. 2020;21(1):152. [14] MITTAL S, GARG B, MEHTA N, et al. Histopathological, Ultrastructural, and Immunohistochemical Findings in Radial Longitudinal Deficiency: A Prospective, Observational Study. J Hand Surg Am. 2022;47(8):789.e1-789.e8. [15] ISHIHARA S, YAMAMOTO H, IWASAKI T, et al. Histological and immunohistochemical features and genetic alterations in the malignant progression of giant cell tumor of bone: a possible association with TP53 mutation and loss of H3K27 trimethylation. Mod Pathol. 2022;35(5):640-648. [16] HU L, XIE X, XUE H, et al. MiR-1224-5p modulates osteogenesis by coordinating osteoblast/osteoclast differentiation via the Rap1 signaling target ADCY2. Exp Mol Med. 2022;54(7):961-972. [17] XU Y, GENG Y, WANG H, et al. Cyclic helix B peptide alleviates proinflammatory cell death and improves functional recovery after traumatic spinal cord injury. Redox Biol. 2023;64:102767. [18] ZHANG Y, ZHANG J, SUN Z, et al. MAPK8 and CAPN1 as potential biomarkers of intervertebral disc degeneration overlapping immune infiltration, autophagy, and ceRNA. Front Immunol. 2023;14:1188774. [19] ZHANG W, ZHOU X, HOU W, et al. Reversing the imbalance in bone homeostasis via sustained release of SIRT-1 agonist to promote bone healing under osteoporotic condition. Bioact Mater. 2022;19:429-443. [20] WANG L, PAN Y, LIU M, et al. Wen-Shen-Tong-Luo-Zhi-Tong Decoction regulates bone-fat balance in osteoporosis by adipocyte-derived exosomes. Pharm Biol. 2023;61(1):568-580. [21] LI T, YUAN J, XU P, et al. PMAIP1, a novel diagnostic and potential therapeutic biomarker in osteoporosis. Aging. 2024;16(4):3694-3715. [22] JI C, DONG Q, LIU H, et al. Acyl-protein thioesterase1 alleviates senile osteoporosis by promoting osteoblast differentiation via depalmitoylation of BMPR1a. Regen Ther. 2023;24:351-360. [23] ARRON JR, CHOI Y. Osteoimmunology-bone versus immune system. Nature. 2000;408(6812):535-536. [24] ANARGYROU K, FOTIOU D, VASSILAKOPOULOS TP, et al. Low Bone Mineral Density and High Bone Turnover in Patients With Non-Hodgkin’s Lymphoma (NHL) Who Receive Frontline Therapy: Results of a Multicenter Prospective Study. Hemasphere. 2019;3(6):e303. [25] Weitzmann MN. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Scientifica (Cairo). 2013;2013:125705. [26] FISCHER V, HAFFNER-LUNTZER M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin Cell Dev Biol. 2022;123:14-21. [27] SUN W, MEEDNU N, ROSENBERG A, et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat Commun. 2018;9(1):5127. [28] CAI S, CHEN Y, HU Z, et al. The landscape of T and B lymphocytes interaction and synergistic effects of Th1 and Th2 type response in the involved tissue of IgG4-RD revealed by single cell transcriptome analysis. J Autoimmun. 2022;133:102944. [29] BASHIROVA AA, ZHENG W, AKDAG M, et al. Population-specific diversity of the immunoglobulin constant heavy G chain (IGHG) genes. Genes Immun. 2021; 22(7-8):327-334. [30] PRAZMA CM, YAZAWA N, FUJIMOTO Y, et al. CD83 expression is a sensitive marker of activation required for B cell and CD4+ T cell longevity in vivo. J Immunol. 2007;179(7):4550-4562. [31] ZHANG H, WANG R, WANG G, et al. Single-Cell RNA Sequencing Reveals B Cells Are Important Regulators in Fracture Healing. Front Endocrinol (Lausanne). 2021;12:666140. [32] VELOUNIAS RL, TULL TJ. Human B-cell subset identification and changes in inflammatory diseases. Clin Exp Immunol. 2022;210(3):201-216. [33] ZHAI Y, CHEN L, ZHAO Q, et al. Cysteine carboxyethylation generates neoantigens to induce HLA-restricted autoimmunity. Science. 2023;379(6637):eabg2482. [34] FENG M, WANG X, ZHOU S, et al. CD83+B cells alleviate uveitis through inhibiting DCs by sCD83. Immunology. 2023;170(1):134-153. [35] XU K, WANG R, XIE H, et al. Single-cell RNA sequencing reveals cell heterogeneity and transcriptome profile of breast cancer lymph node metastasis. Oncogenesis. 2021;10(10):66. [36] PASELLO M, MANARA MC, SCOTLANDI K. CD99 at the crossroads of physiology and pathology. J Cell Commun Signal. 2018;12(1):55-68. [37] LI L, DAI F, WANG L, et al. CCL13 and human diseases. Front Immunol. 2023;14:1176639. [38] LIN F, XUE DT, XIE T, et al. HMGB1 promotes cellular chemokine synthesis and potentiates mesenchymal stromal cell migrationvia Rap1 activation. Mol Med Rep. 2016;14(2):1283-1289. [39] WU X, LIU Y, JIN S, et al. Single-cell sequencing of immune cells from anticitrullinated peptide antibody positive and negative rheumatoid arthritis. Nat Commun. 2021;12(1):4977. [40] DU Y, CAI Y, LV Y, et al. Single-cell RNA sequencing unveils the communications between malignant T and myeloid cells contributing to tumor growth and immunosuppression in cutaneous T-cell lymphoma. Cancer Lett. 2022;551:215972. [41] LI H, HE Z, DENG B, et al. Cytokines and chemokines involved in HLA-B27-positive ankylosing spondylitis-associated acute anterior uveitis. Mol Vis. 2023;29:378-385. [42] CIECHANOWSKA A, MIKA J. CC Chemokine Family Members’ Modulation as a Novel Approach for Treating Central Nervous System and Peripheral Nervous System Injury-A Review of Clinical and Experimental Findings. Int J Mol Sci. 2024;25(7):3788. [43] HUANG Y, HUANG J, ZHANG P, et al. Integrated analysis of hub gene expression in multiple myeloma. J BUON. 2021;26(5):2040-2052. [44] PANG J, YU Q, CHEN Y, et al. Integrating Single-cell RNA-seq to construct a Neutrophil prognostic model for predicting immune responses in non-small cell lung cancer. J Transl Med. 2022;20(1):531. [45] ZHANG Q, YU B, ZHANG Y, et al. Combination of single-cell and bulk RNA seq reveals the immune infiltration landscape and targeted therapeutic drugs in spinal cord injury. Front Immunol. 2023;14:1068359. |
| [1] | Zhou Jinhai, Li Jiangwei, Wang Xuquan, Zhuang Ying, Zhao Ying, Yang Yuyong, Wang Jiajia, Yang Yang, Zhou Shilian. Three-dimensional finite element analysis of anterior femoral notching during total knee arthroplasty at different bone strengths [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1775-1782. |
| [2] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [3] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [4] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [5] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [6] | Li Yueyao, Zhang Min, Yang Jiaju. Cistanoside A mediates p38/MAPK pathway to inhibit osteoclast activity [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1144-1151. |
| [7] | Zheng Lin, Jin Wenjun, Luo Shanshan, Huang Rui, Wang Jie, Cheng Yuting, An Zheqing, Xiong Yue, Gong Zipeng, Liao Jian. Eucommia ulmoides promotes alveolar bone formation in ovariectomized rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1159-1167. |
| [8] |
Huang Xiaobin, Ge Jirong, Li Shengqiang, Xie Lihua, Huang Jingwen, He Yanyan, Xue Lipeng.
Mechanisms of different yin nourishing and kidney tonifying methods on osteoclastysis pathway in ovariectomized rats #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1214-1219.
|
| [9] | Qian Kun, Li Ziqing, Sun Shui . Endoplasmic reticulum stress in the occurrence and development of common degenerative bone diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1285-1295. |
| [10] | Lan Shuangli, Xiang Feifan, Deng Guanghui, Xiao Yukun, Yang Yunkang, Liang Jie. Naringin inhibits iron deposition and cell apoptosis in bone tissue of osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 888-898. |
| [11] | Wang Dongyang, Yang Qiaohui, Lin Xinchao. Relationship between vitamin D levels and reproductive characteristics and exercise dietary situation in postmenopausal women [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1021-1025. |
| [12] | Zhang Lichuang, Yang Wen, Ding Guangjiang, Li Peikun, Xiao Zhongyu, Chen Ying, Fang Xue, Zhang Teng. Dispersion effect of bone cement after vertebroplasty using individualized unilateral external pedicle approach and bilateral pedicle approach [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 800-808. |
| [13] | Zhang Xiongjinfu, Chen Yida, Cheng Xinyi, Liu Daihui, Shi Qin . Exosomes derived from bone marrow mesenchymal stem cells of young rats to reverse senescence in aged rat bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7709-7718. |
| [14] | Wang Rongqiang, Yang Liu, Wu Xiangkun, Shang Lilin. Analysis of factors associated with prognosis of osteoporosis patients after hip arthroplasty and construction of Nomogram prediction model [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(33): 7137-7142. |
| [15] | Yan Jinlian, Xu Zhengquan, Wei Renjie, Wang Yehua. Hip joint function recovery and prediction model construction after proximal femoral nail antirotation for intertrochanteric fractures [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(33): 7189-7195. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||