Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (16): 3494-3502.doi: 10.12307/2025.437
Previous Articles Next Articles
Hernia repair patch: recent advances in material design and application
Chen Jingyu, Hong Ge, Guo Ning, Liu Tianjun
- 1Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China; 2Tianjin Key Laboratory of Biomedical Materials, Institute of Biomedical Engineering, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin 300192, China; 3School of Basic Medical Sciences, Hebei University, Baoding 071000, Hebei Province, China
-
Received:2024-03-25Accepted:2024-05-25Online:2025-06-08Published:2024-09-06 -
Contact:Liu Tianjun, Researcher, Tianjin Key Laboratory of Biomedical Materials, Institute of Biomedical Engineering, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin 300192, China -
About author:Chen Jingyu, Master, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China -
Supported by:Capital Health Research & Development of Special Fund Program, No. 2022-1-4041 (to LTJ); Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, No. 2021-I2M-1-052 (to HG)
CLC Number:
Cite this article
Chen Jingyu, Hong Ge, Guo Ning, Liu Tianjun. Hernia repair patch: recent advances in material design and application[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3494-3502.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
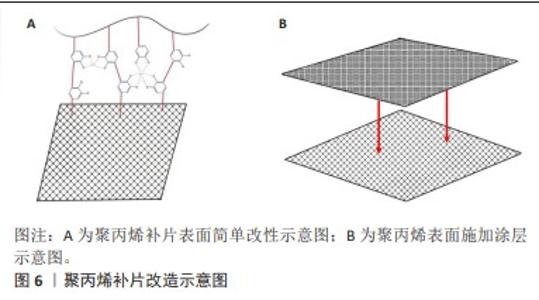
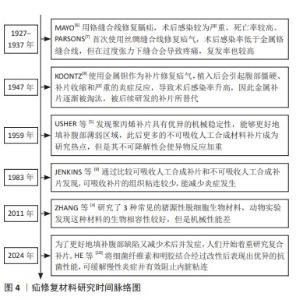
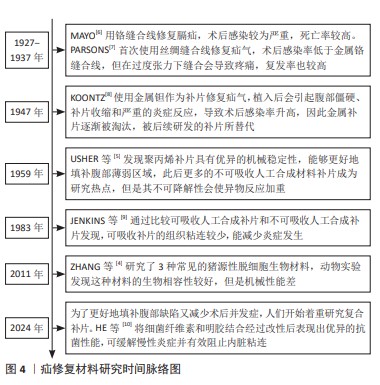
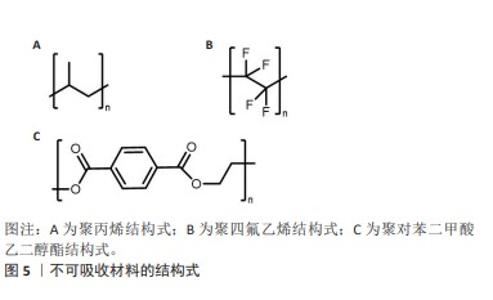
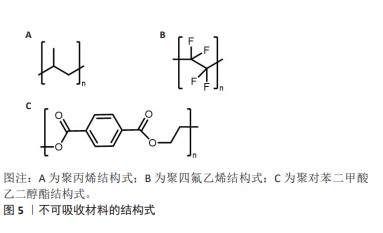
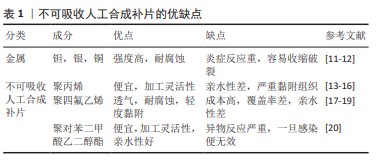
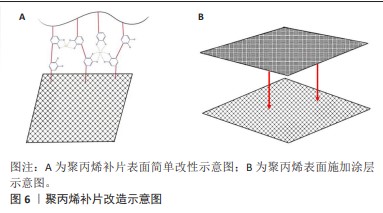
2.2.1 聚丙烯 在1949年,聚丙烯被引入作为疝修复手术的材料[21],直至今日,它依然被视作该领域内的“黄金标准”[22]。聚丙烯作为一种热塑性材料具有良好的机械性能、化学稳定性和耐化学腐蚀性等特点,但具有较高的收缩率。聚丙烯类补片便宜、容易获得,而且还具有良好的柔韧性和弹性[23], 但是常常会发生异物反应和炎症。尽管有一些研究表明,聚丙烯补片在炎症早期(3-5 d)能够抵抗粘连的特性[24],但在愈合过程后期,特别是21 d后仍有可能出现显著的腹部粘连问题,尤其是在小肠区域。这种粘连现象可能引发一系列长期症状,如持续性疼痛、肠梗阻、纤维化[25]、术后感染和瘘管形成[26]。因此,尽管聚丙烯补片在短期内具有一定的抗粘连效果,但长期应用仍然需要密切监测和适当的管理以预防相关并发症的发生。为了解决基于聚丙烯材料的感染和组织黏附方面的限制,已经开发出多种有效的策略,QIAO等[27]通过组装单宁酸和Fe3+形成的金属-酚醛网络对聚丙烯补片进行简单改性(图6A),实现了良好的抗炎效果,同时也降低了胶原蛋白在补片周围的沉积量。这类补片是通过对聚丙烯补片选择性改性来达到理想的效果,另一种策略是在聚丙烯补片表面通过静电纺丝的技术直接施加一种涂层(图6B),这样可以有效提高材料的附着力,同时减少材料与生物组织接触时产生的异物反应[28]。"
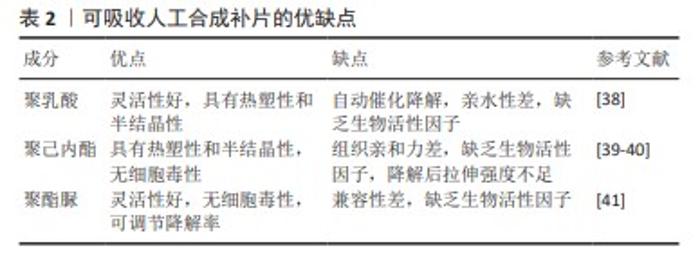
2.2.2 聚四氟乙烯 聚四氟乙烯是一种由四氟乙烯单体聚合而成的热塑性材料,具有无毒性、化学稳定性以及天然的抗菌性质。此外,与聚丙烯类材料相比,聚四氟乙烯材料引发的炎症反应、组织纤维生长及细胞黏附程度较低,这种现象主要归因于聚四氟乙烯的微孔结构能够有效减缓细菌的渗透,同时允许免疫系统中的巨噬细胞穿过[29],这种结构有助于减少身体对植入物的免疫反应,减轻异物反应。聚四氟乙烯在疝修复术中的应用也存在一些局限性,主要体现在其机械强度不高以及与宿主组织融合不充分,这种情况可能会导致组织愈合不完全,进而影响修复效果的持久性和稳定性。膨体聚四氟乙烯是一种由聚四氟乙烯树脂通过特定的生产加工工艺膨胀形成的材料,在强度、抗粘连性和微孔率方面都优于聚四氟乙烯,是一种非常有价值的补片材料。üNEK等[30]对接受膨体聚四氟乙烯的156例患者进行了长达119个月的临床追踪研究,结果表明该类型补片能够提供安全且持久的疝修复效果。但是膨体聚四氟乙烯补片的感染风险相对较高,并且成本也较为昂贵;此外,膨体聚四氟乙烯疝气贴片的覆盖面积有限,这可能导致在进行腹壁重建手术时无法完全覆盖需要修复的区域。为了克服这些挑战,研究者们已经开发了多种策略来改善膨体聚四氟乙烯的性能,其中一种常见的方法是通过化学浸渍技术将其他物质引入到材料中[31],通过共价或非共价键将特定的化学物质固定在材料表面或内部,以增强其生物相容性和抗感染能力[32];另一种方法是界面修饰,涉及到改变材料表面的化学性质或物理结构,以使其更加亲水或亲细胞,从而促进组织生长和愈合,如可以通过等离子体处理[33]、表面粗糙化或添加特定的生物活性分子来实现界面修饰。 2.2.3 聚对苯二甲酸乙二醇酯 聚对苯二甲酸乙二醇酯是通过对苯二甲酸和乙二醇进行缩合反应合成的一种聚合物,这种材料自1939年被首次研发以来,因其生物相容性、易于合成和可改性在医疗领域得到广泛应用[34-35]。虽然聚对苯二甲酸乙二醇酯的价格较为经济实惠,但是其机械稳定性仅相当于聚丙烯的1/3,并且在低应力条件下拉伸弹性较差[35]。聚对苯二甲酸乙二醇酯类补片的组织相容性及顺应性不如聚丙烯类补片,这可能会引起瘢痕收缩增加,但在降低术后感染风险和疾病复发方面,聚对苯二甲酸乙二醇酯显示出与聚丙烯相当甚至更为优异的性能[20,34]。医学检查结果表明,聚对苯二甲酸乙二醇酯补片存在许多不足之处,例如胶原蛋白的合成和积累减少、愈合组织中羟脯氨酸含量下降[36],以及比聚丙烯补片更显著的纤维化现象。传统的聚对苯二甲酸乙二醇酯补片由于其复丝结构限制了巨噬细胞等免疫细胞的迁移,从而影响了其抗感染能力。为了克服这一问题,单丝结构类的聚对苯二甲酸乙二醇酯补片被开发出来[34],解决聚对苯二甲酸乙二醇酯限制的另一种方法是开发含有纳米聚酯纤维的支架和表面经过改性的聚对苯二甲酸乙二醇酯材料[37]。 2.3 可吸收人工合成补片 在疝修复手术中,采用可吸收补片正成为一种新兴的做法。可吸收补片在人体内会随着时间逐步被分解并被身体组织所吸收,它们在植入后为受损组织提供必要的支持,直到新的健康组织形成并接管了补片的功能(表2)。这种材料的应用为疝气修复提供了一种创新的解决方案,有望加快术后恢复过程并降低长期并发症的风险。 "

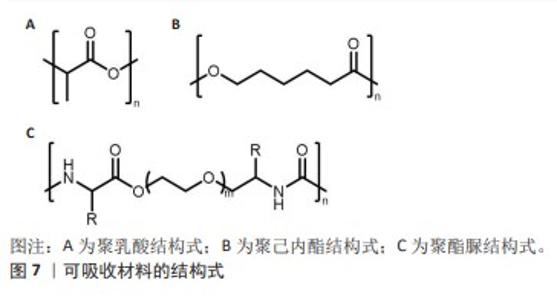
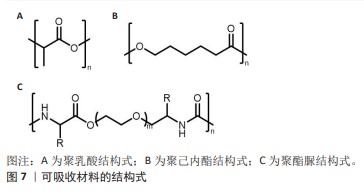
2.3.1 聚乳酸 聚乳酸(结构式见图7A)是一种由乳酸聚合而成的高分子聚合物,无毒,可生物降解,并且能被人体代谢吸收。聚乳酸除了具有生物可降解性外,还能够抑制组织粘连,降低成纤维细胞分泌炎性因子,促进成纤维细胞增殖,并且降解速率慢于组织重塑[42],这意味着聚乳酸补片可以在不干扰正常愈合过程的情况下逐渐被吸收和替换。聚乳酸补片的应用显著降低了盲肠和腹壁之间的粘连,在2005年AVITAL等[43]就已经发现使用聚乳酸补片能将小鼠的术后粘连发生率从71.4%降低到10.5%。聚乳酸常与其他聚合物混合使用,使其有更好的机械强度,将聚乳酸、聚氨酯和脱细胞真皮基质粉末相结合能够提供疝修补所需的高效机械强度(16 N/cm),并且机械强度值高于聚丙烯补片[38]。 2.3.2 聚己内酯 聚己内酯(结构式见图7B)是一种合成有机高分子材料,无细胞毒性,应用于疝修复领域能够避免因纤维化导致的并发症[39],因此这种材料展现出了更大的应用潜力和更贴合人性化的需求。相较于聚乳酸,聚己内酯有着更长的降解周期[44],在疝修复领域提供长期机械支撑方面表现出了更好的适用性。纯聚己内酯具有一定的脆性,经常与其他材料混合以改善其性能并作为疝修复补片的替代品,如将聚乳酸和聚己内酯进行共聚反应可以得到一种新型材料,即聚(l-丙交酯-co-ε-己内酯)[45],这种共聚物结合了2种单体的特性,从而在保持聚己内酯生物降解性的同时增加了聚乳酸的强度和刚性,使得聚(l-丙交酯-co-ε-己内酯)成为一种在疝修复应用中更具吸引力的材料选择。 2.3.3 聚酯脲 聚酯脲(结构式见图7C)是一种新型的生物降解高分子材料,为传统的合成疝修复材料提供了更环保的选择[44]。聚酯脲的侧链能够与氨基酸的R集团相连接,这一特性使其具有极大的灵活性,通过调整这些侧链的化学组成能够实现对材料性能的精确控制,以适应疝修复手术的各种场景。DREGER等[41]于2018年探讨了基于线性与支链形式的L-缬氨酸改性聚酯脲的效果,发现这些聚酯脲材料无细胞毒性,并且在经过灭菌处理之后有效限制了炎症反应。通过两性离子氨基酸处理过的聚酯脲补片能够降低纤维蛋白原的吸附性[46],这表明两性离子功能化的聚酯脲材料可降低黏附的程度和韧性。聚酯脲的体内降解速度是可以进行调节的[47],以便与疝修复过程中组织愈合的进程相对应。要实现这一目标,关键在于二醇链的长度以及合成过程中用于R基团的氨基酸类型[41,47-48],DREGER等[46]的降解测试结果表明,含有2% L-缬氨酸的聚酯脲在体内经过3个月后能够展现出机械切割的效果,这说明该材料能够在疝修补手术后提供长期的强度支持,以抵抗腹内压,这一发现支持了先前关于聚酯脲性能受到其支链结构以及聚合物链流动性调控的观点。尽管如此,为了更深入地了解聚酯脲作为疝修补材料的潜力,还需要进行更多的研究,包括评估其在不同条件下的生物相容性、长期降解行为以及对人体组织的影响等,通过这些研究可以更好地确定聚酯脲在临床应用中的有效性和安全性。"
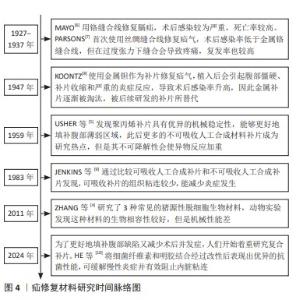
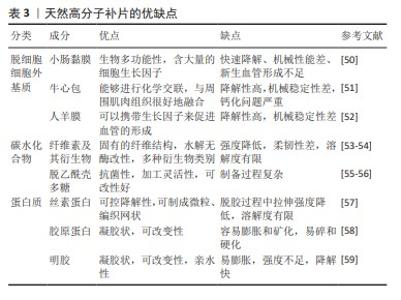
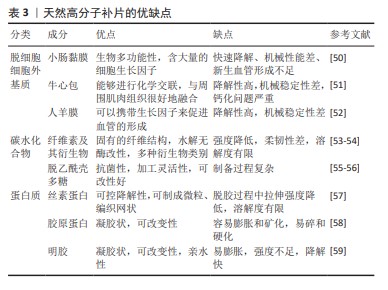
2.4.1 脱细胞细胞外基质 脱细胞细胞外基质是一种独特的生物材料,具有精确和完整的3D空间结构以及与真皮组织的生物相容性,可去除细胞的高免疫原性组成,同时保留了细胞外基质及其基底膜的细胞成分[60-61]。使用脱细胞细胞外基质制成的疝修复补片,已经显示出作为疝修复生物材料的巨大潜力[62]。1995,PREVEL等[63]首次对脱细胞细胞外基质补片的抗炎与抗黏附特性进行评估,此后小肠黏膜便开始应用于疝修补手术中。后续临床研究证实,小肠黏膜能够有效促进细胞外基质内部的血管新生[64-65]。然而,这种小肠黏膜生物补片的降解速率较快[65],可能导致补片逐渐失去结构稳定性,进而引起腹壁的弱化、突出以及疝气的重新出现等问题。20世纪末,牛心包开始被用作疝修复补片[66]。近期研究比较了2种不同处理的牛心包补片:未经化学交联的牛心包补片与经过戊二醛交联的牛心包补片[51,66],发现戊二醛处理的牛心包补片表现出优异的强度、稳定性,并且能够很好地与周围肌肉组织融合。YU等[48]研究探索了使用氧化透明质酸作为牛心包补片交联的新型介质,相比于传统的戊二醛交联方法,氧化透明质酸交联的牛心包补片具有更高的柔韧性。人羊膜是胎盘的一部分,脱细胞后能够有效抑制转化生长因子β1的表达,同时促进新血管的形成,加之其卓越的宿主组织整合能力和高度的生物相容性,因此在临床应用方面被认为比小肠黏膜更为优越[52]。1994年,H.O.及其团队利用人羊膜成功制备了一种用于预防组织粘连的物理性隔离层[67];2000年,SZABO等[68]在大鼠腹疝实验中采用人羊膜的聚丙烯补片进行了实验研究,这些研究结果显示粘连形成的平均数和面积比例均趋向于零,表明人羊膜在减少术后粘连形成方面是有效的。然而人羊膜的机械强度不足,众多关于人羊膜的研究工作都集中于开发基于人羊膜的复合材料,以用于疝修补[52]。通过将人羊膜与其他材料结合旨在提高其整体性能,包括增强其耐用性和稳定性,以便更好地满足临床疝气修复的需求。 2.4.2 碳水化合物 近些年,细菌纤维素因其独特的性质而在疝气修复材料的研发上备受关注[69]。相较于传统的纤维素材料,以细菌纤维素为基础的疝修复补片具有更高的结晶度和更好的内聚性,这些特性使得其在疝修复手术中能够提供更为安全和有效的方法[22]。除此之外,细菌纤维素是由细菌产生的一种碳水化合物,不仅毒性低,还具有良好的水溶性和生物可降解性,对环境友好[54]。通过在不同生长条件下调节细菌纤维素的分子结构可以实现对其物理性能如柔韧性和稳定性的优化。总之,根据不同的应用需求,以细菌纤维素为材料的疝修复补片展现出了极大的可调性,这使得它们既能够用于短期修复也能够用于长期植入。壳聚糖是由甲壳素经过脱乙酰化处理后得到的产物,具有良好抗菌效果和生物降解能力,在组织再生医学领域得到了广泛运用[55]。研究指出,使用壳聚糖基材料的疝修复产品在血液相容性方面存在不足[70],可能导致急性和慢性炎症反应,进而影响伤口愈合。此外,壳聚糖及其衍生物通常表现出疏水性,并且在水中的溶解度有限,这对于水性应用构成挑战[55]。然而,类似细胞外基质的甲壳素及其衍生物在辅助疝修复中作为载体或补片被广泛采用[71],主要是因为它们具备诸如吸水性、保湿性、生物降解性等特殊功能,以及能够对环境温度和pH值做出响应的特性[55-56],例如羧甲基化壳聚糖是壳聚糖的水溶性衍生物,常用于疝修复,因为它能促进细胞增殖、抑制瘢痕形成并加快伤口愈合过程[72]。 2.4.3 蛋白质 丝素蛋白是一种具有多功能性的生物材料,它可以呈现为颗粒、再生纤维、薄膜、凝胶和多孔基质等多种形态[73],因其杰出的生物相容性、低细胞毒性、非免疫原性和对抗蛋白水解的能力而被广泛应用于多个领域[74]。2014年,CLEMENS等[75]采用丝素蛋白生物支架来增强患者的腹壁组织,通过对2011-2013年间接受丝素蛋白生物支架装置患者的回顾性分析,评估了丝素蛋白生物支架在支撑软组织和腹壁修复方面的有效性,结果显示丝素蛋白生物支架装置在腹壁修复方面是安全有效的。目前,丝素蛋白依然是腹壁修复中使用的理想材料[76]。人体中的胶原蛋白含量占人体总蛋白质的1/4以上,并且在哺乳动物的皮肤、肌腱等组织中普遍存在,在促进组织恢复和愈合方面发挥着不可或缺的作用。在腹壁的细胞外基质里,胶原蛋白是最主要的成分[77],因此,基于胶原蛋白的疝修补材料在腹壁修复领域具有巨大潜力。但由于胶原蛋白原材料获取、提取纯化过程的不确定性,以及维持机械强度和物理化学特性的挑战[78],导致其研发进程缓慢[79],因此对于胶原蛋白类补片的开发仍需进一步强化。HE等[58]制备了胶原蛋白聚集体(Col-A)和胶原蛋白分子(Col-M)2种支架,Col-A支架相较于Col-M支架展现出更高的柔韧性以及更优的孔隙率和强度,并且Col-A支架在腹壁组织再生方面具有显著效果,这一结论得到了多个生物学指标的支持,包括肌肉层厚度的增加、肌纤维面积比例的提高、结蛋白表达细胞的增多,以及大鼠体内毛细血管密度的提升,这些观察结果均指向Col-A支架在促进腹壁组织修复和再生方面的潜力。明胶是胶原蛋白的一种热变性产品,它通过一个不可逆的过程形成,并且在某些方面与原生胶原蛋白相似。在医疗健康和生物材料的领域里,明胶经常被用作胶原蛋白的代替品[80-82]。众多研究已经证实,明胶可以用作物理隔离层或者具备特定功能的凝胶状支架,因此被广泛应用于各种混合型的疝修补物料中。DYDAK等[69]探讨了功能化肌腱明胶的效果,其中明胶被改性加入了2-脲基-4[1H]-嘧啶酮单元(UPy单元),即TGUPy。TGUPy是一种能够根据温度变化从溶胶转变为凝胶的黏合剂,利用温敏性的氢键作用有效预防术后腹壁与盲肠之间的粘连。TGUPy在人体正常体温以上时保持溶胶状态,因而具有很好的注射性,能够附着于受损组织表面,促进伤口的愈合。 2.5 复合补片 尽管在不可吸收人工合成补片和可吸收人工合成补片方面取得了显著进展,这些补片分别提供了更高的机械强度和更好的生物相容性及降解性,但与补片植入相关的一些并发症如补片收缩、慢性炎症以及与术后感染等问题依然普遍存在。这些问题需要通过进一步的研究和技术创新来解决,以改善患者的治疗效果和生活质量。在这种情况下,采用不可吸收补片作为基础,将其与一种或多种合成或天然材料结合(例如聚己内酯、丝素蛋白和抗菌药物等)可以制造出复合材料补片,这种复合材料补片继承了传统不可吸收补片优良的机械性能,同时通过可吸收部分的设计降低了一些并发症发生的风险。最初应用于疝气修复的复合补片是在1999年由巴德(Bard)公司研制的聚丙烯-膨体聚四氟乙烯补片[83],这一材料的成功激发了后续对更多种类复合修补材料的探索,包括水凝胶、纳米纤维膜以及表面涂层和负载药物的复合补片等。为了减少蛋白质吸附和炎症的发生,QIAO等[84]制备出了由多巴胺介导的两性离子涂层,能够在保证聚丙烯补片机械强度的情况下减少巨噬细胞的黏附和促炎因子的释放;HU等[85]将3D打印技术和静电纺丝相结合,利用聚己内酯、聚乙烯醇和大豆肽制备出新型可生物降解补片,聚己内酯作为补片的机械支撑,聚乙烯醇和大豆肽作为抗粘连层有效解决了腹腔粘连的问题;LUAN等[86]采用静电纺丝技术成功地将丝素蛋白纤维膜附着于聚丙烯网上,从而结合了丝素蛋白的优点和聚丙烯的特性,通过将该补片用于动物实验发现,术后第3天和第7天几乎未观察到组织粘连现象,仅有部分大鼠在术后第14天和第90天出现了轻微粘连;此外,该类补片还能促进新血管的形成。术后感染是疝修补手术中常见的问题,可能导致伤口愈合受阻并增加再次手术的需求。为了解决这一问题,将抗菌药物整合到补片中提供了一种有效的解决方案。有研究指出,将经过乙酰乙酰基修饰后并载有庆大霉素的棉织物与聚丙烯补片进行简单缝合,可同时实现抑制感染和避免术后组织粘连的效果[87]。尽管载药复合补片具有相当的抗感染功效,但尚未解决的低安全性和高成本问题促使研究者开发抗菌疝修复补片的替代品。基于金属离子或金属氧化物的生物功能特性,提出了一些解决方案来提高补片的抗感染稳定性。研究人员已经探索了利用金属离子的生物活性特性来提升组织修复补片在抗菌方面的持久效能,ZHANG等[88]通过向小肠黏膜中引入Cu+开发出一种新型的生物修复材料,证实这种改良补片展现了极高的杀菌能力、良好的细胞相容性,并能刺激血管内皮生长因子信号通路的激活,从而促进了新血管的生长和胶原蛋白的成熟。以上这些积极的成果预示,这类补片在防止粘连、抑菌以及加速组织的自然愈合过程中具有巨大的潜力(表4)。 "
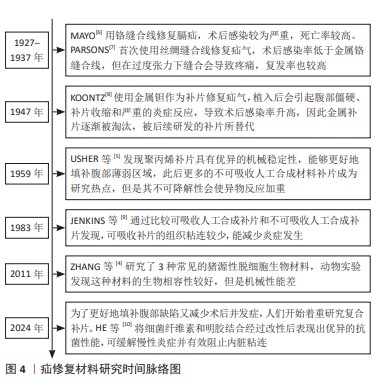
| [1] XU LS, LI Q, WANG Y, et al. Current status and progress of laparoscopic inguinal hernia repair: A review. Medicine (Baltimore). 2023;102(31): e34554. [2] SHAKIL A, APARICIO K, BARTA E, et al. Inguinal Hernias: Diagnosis and Management. Am Fam Physician. 2020;102(8):487-492. [3] KUSHNER BS, AREFANIAN S, MCALLISTER J, et al. Examination of abdominal wall perfusion using varying suture techniques for midline abdominal laparotomy closure. Surg Endosc. 2022;36(6):3843-3851. [4] ZHANG J, WANG GY, XIAO YP, et al. The biomechanical behavior and host response to porcine-derived small intestine submucosa, pericardium and dermal matrix acellular grafts in a rat abdominal defect model. Biomaterials. 2011;32(29):7086-7095. [5] USHER FC, HILL JR, OCHSNER JL. Hernia repair with Marlex mesh. A comparison of techniques. Surgery. 1959;46:718-724. [6] MAYO CH. Repair of hernia of the diaphragm. Ann Surg. 1927;86(4): 481-484. [7] PARSONS WB. Silk sutures in the repair of hernia. Ann Surg. 1937; 106(3):343-347. [8] KOONTZ AR. Preliminary report on the use of tantalum mesh in the repair of ventral hernias. Trans South Surg Assoc. 1947;59(1 vol.): 382-388. [9] JENKINS SD, KLAMER TW, PARTEKA JJ, et al. A comparison of prosthetic materials used to repair abdominal wall defects. Surgery. 1983;94(2): 392-398. [10] HE P, WANG D, ZHENG R, et al. An antibacterial biologic patch based on bacterial cellulose for repair of infected hernias. Carbohydr Polym. 2024;333:121942. [11] BALL L. The repair of inguinal hernia and the use of filigrees. Br J Surg. 1958;45(193):562-564. [12] BURKE GL. The corrosion of metals in tissues; and an introduction to tantalum. Can Med Assoc J. 1940;43(2):125-128. [13] CALIS H, SENGUL S, GULER Y, et al. A Novel Transabdominal Preperitoneal Hernioplasty Technique in the Repair of Large Inguinal Hernias: The use of Polypropylene Mesh in the form of Plug-patch. J Coll Physicians Surg Pak. 2021;30(7):825-828. [14] KNIGHT KM, KING GE, PALCSEY SL, et al. Mesh deformation: A mechanism underlying polypropylene prolapse mesh complications in vivo. Acta Biomater. 2022;148:323-335. [15] YANG S, SHEN YM, WANG MG, et al. Titanium-coated mesh versus standard polypropylene mesh in laparoscopic inguinal hernia repair: a prospective, randomized, controlled clinical trial. Hernia. 2019;23(2): 255-259. [16] YE Z, ZHANG L, LIU T, et al. The effect of surface nucleation modulation on the mechanical and biocompatibility of metal-polymer biomaterials. Front Bioeng Biotechnol. 2023;11:1160351. [17] RUHRNSCHOPF CG, REUSMANN A, BOGLIONE M, et al. Biological versus synthetic patch for the repair of congenital diaphragmatic hernia: 8-year experience at a tertiary center. J Pediatr Surg. 2021;56(11):1957-1961. [18] NICOLAU AE, VASILE R, HAIDUCU C. Laparoscopic Repair of Small Ventral Hernias Using the “Ventralex™ Hernia Patch”. Chirurgia (Bucur). 2019;114(1):95-102. [19] VAN DER LEI B, BLEICHRODT RP, SIMMERMACHER RK, et al. Expanded polytetrafluoroethylene patch for the repair of large abdominal wall defects. Br J Surg. 1989;76(8):803-805. [20] O’DWYER P, CHEW C, QANDEEL H. Long term outcome and elasticity of a polyester mesh used for laparoscopic ventral hernia repair. Hernia. 2022;26(2):489-493. [21] SAHA T, WANG X, PADHYE R, et al. A review of recent developments of polypropylene surgical mesh for hernia repair OpenNano. 2022;7: 100046. [22] CUI T, YU F, ZHANG Q, et al. Double-layered bacterial cellulose mesh for hernia repair. Colloid Interface Sci Commun. 2021;44:100496. [23] LIU T, YE Z, YU B, et al. Biomechanical behaviors and visco-hyperelastic mechanical properties of human hernia patches with polypropylene mesh. Mech Compos Mater. 2023;176:104529. [24] HU W, ZHANG Z, ZHU L, et al. Combination of Polypropylene Mesh and in Situ Injectable Mussel-Inspired Hydrogel in Laparoscopic Hernia Repair for Preventing Post-Surgical Adhesions in the Piglet Model. ACS Biomater Sci Eng. 2020;6(3):1735-1743. [25] ARTSEN AM, RYTEL M, LIANG R, et al. Mesh induced fibrosis: The protective role of T regulatory cells. Acta Biomater. 2019;96:203-210. [26] PANDE T, NAIDU CS. Mesh infection in cases of polypropylene mesh hernioplasty. Hernia. 2020;24(4):849-856. [27] QIAO Y, ZHANG Q, WANG Q, et al. Synergistic Anti-inflammatory Coating “Zipped Up” on Polypropylene Hernia Mesh. ACS Appl Mater Interfaces. 2021;13(30):35456-35468. [28] AYDEMIR SEZER U, SANKO V, GULMEZ M, et al. A polypropylene-integrated bilayer composite mesh with bactericidal and antiadhesive efficiency for hernia operations. ACS Biomater Sci Eng. 2017;3(12): 3662-3674. [29] XU D, FANG M, WANG Q, et al. Latest Trends on the Attenuation of Systemic Foreign Body Response and Infectious Complications of Synthetic Hernia Meshes. ACS Appl Bio Mater. 2022;5(1):1-19. [30] ÜNEK T, SÖKMEN S, EGELI T, et al. The results of expanded-polytetrafluoroethylene mesh repair in difficult abdominal wall defects. Asian J Surg. 2019;42(1):131-143. [31] TALON I, SCHNEIDER A, BALL V, et al. Functionalization of PTFE Materials Using a Combination of Polydopamine and Platelet-Rich Fibrin. J Surg Res. 2020;251:254-261. [32] ROINA Y, AUBER F, HOCQUET D, et al. ePTFE functionalization for medical applications. Mater Today Chem. 2021;20:100412. [33] NIAN S, KEARNS VR, WONG DSH, et al. Plasma polymer surface modified expanded polytetrafluoroethylene promotes epithelial monolayer formation in vitro and can be transplanted into the dystrophic rat subretinal space. J Tissue Eng Regen Med. 2021;15(1):49-62. [34] XU D, FANG M, WANG Q, et al. Latest trends on the attenuation of systemic foreign body response and infectious complications of synthetic hernia meshes. ACS Appl Bio Mater. 2021;5(1):1-19. [35] BERREVOET F, DOERHOFF C, MUYSOMS F, et al. A multicenter prospective study of patients undergoing open ventral hernia repair with intraperitoneal positioning using the monofilament polyester composite ventral patch: interim results of the PANACEA study. Med Devices (Auckl). 2017;10:81-88. [36] AKCAKAYA A, AYDOGDU I, CITGEZ B. Investigation into the optimal prosthetic material for wound healing of abdominal wall defects. Exp Ther Med. 2018;15(2):1622-1625. [37] RODRÍGUEZ M, GÓMEZ-GIL V, PÉREZ-KÖHLER B, et al. Polymer hernia repair materials: Adapting to patient needs and surgical techniques. Materials (Basel). 2021;14(11):2790. [38] HU Q, ZHANG R, ZHANG H, et al. Topological Structure Design and Fabrication of Biocompatible PLA/TPU/ADM Mesh with Appropriate Elasticity for Hernia Repair. Macromol Biosci. 2021;21(6):e2000423. [39] CESUR O, TANIR TE, CELEPLI P, et al. Enhancing esophageal repair with bioactive bilayer mesh containing FGF. Sci Rep. 2021;11(1):19203. [40] ARIF ZU, KHALID MY, NOROOZI R, et al. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int J Biol Macromol. 2022;218:930-968. [41] DREGER NZ, WANDEL MB, ROBINSON LL, et al. Preclinical in vitro and in vivo assessment of linear and branched l-valine-based poly (ester urea) s for soft tissue applications. ACS Biomater Sci Eng. 2018;4(4): 1346-1356. [42] WANG X, LIU C, LI X, et al. A novel electrospun polylactic acid silkworm fibroin mesh for abdominal wall hernia repair. Mater Today Bio. 2024; 24:00915. [43] AVITAL S, BOLLINGER TJ, WILKINSON JD, et al. Preventing intra-abdominal adhesions with polylactic acid film: an animal study. Dis Colon Rectum. 2005;48:153-157. [44] KIRILLOVA A, YEAZEL TR, ASHEGHALI D, et al. Fabrication of biomedical scaffolds using biodegradable polymers. Chem Rev. 2021;121(18): 11238-11304. [45] GAO R, KONG P, YANG C, et al. Gelatinized PLCL Electrospun Membrane for the Prevention of Postoperative Abdominal Adhesion Through Fibrinolysis Activation. Adv Mater Interfaces. 2022;9(18):2200063. [46] DREGER NZ, ZANDER ZK, HSU YH, et al. Zwitterionic amino acid-based Poly(ester urea)s suppress adhesion formation in a rat intra-abdominal cecal abrasion model. Biomaterials. 2019;221:119399. [47] WADE MB, RODENBERG E, PATEL U, et al. Influence of sterilization technologies on electrospun poly (ester urea) s for soft tissue repair. Biomacromolecules. 2016;17(10):3363-3374. [48] YU J, LIN F, LIN P, et al. Phenylalanine-based poly (ester urea): synthesis, characterization, and in vitro degradation. Macromolecules. 2014;47(1):121-129. [49] COSTA A, ADAMO S, GOSSETTI F, et al. Biological scaffolds for abdominal wall repair: future in clinical application? Materials (Basel). 2019;12(15):2375. [50] JIANG W, ZHANG J, LV X, et al. Use of small intestinal submucosal and acellular dermal matrix grafts in giant omphaloceles in neonates and a rabbit abdominal wall defect model. J Pediatr Surg. 2016;51(3): 368-373. [51] ZHAO Y, LI Y, PENG X, et al. Feasibility study of oxidized hyaluronic acid cross-linking acellular bovine pericardium with potential application for abdominal wall repair. Int J Biol Macromol. 2021;184:831-842. [52] LIU Z, ZHU X, ZHU T, et al. Evaluation of a biocomposite mesh modified with decellularized human amniotic membrane for intraperitoneal onlay mesh repair. ACS Omega. 2020;5(7):3550-3562. [53] SULTANA T, GWON JG, LEE BT. Thermal stimuli-responsive hyaluronic acid loaded cellulose based physical hydrogel for post-surgical de novo peritoneal adhesion prevention. Mater Sci Eng C. 2020;110:110661. [54] RAUCHFUß F, HELBLE J, BRUNS J, et al. Biocellulose for incisional hernia repair—an experimental pilot study. Nanomaterials. 2019;9(2):236. [55] HU H, SUN H, JIANG Z, et al. Study on repair of abdominal wall defect rats with hernia mesh coated with chitosan-based photosensitive hydrogel. Carbohydr Polym. 2022;291:119577. [56] WANG Y, PANG X, LUO J, et al. Naproxen nanoparticle-loaded thermosensitive chitosan hydrogel for prevention of postoperative adhesions. ACS Biomater Sci Eng. 2019;5(3):1580-1588. [57] ZHANG H, LIU Y, CHEN C, et al. Responsive drug-delivery microcarriers based on the silk fibroin inverse opal scaffolds for controllable drug release. Appl Mater Today. 2020;19:100540. [58] HE X, LI W, LIU S, et al. Fabrication of high-strength, flexible, porous collagen-based scaffolds to promote tissue regeneration. Mater Today Bio. 2022;16:100376. [59] NISHIGUCHI A, ICHIMARU H, ITO S, et al. Hotmelt tissue adhesive with supramolecularly-controlled sol-gel transition for preventing postoperative abdominal adhesion. Acta Biomater. 2022;146:80-93. [60] MA S, MA B, YANG Y, et al. Functionalized 3D Hydroxyapatite Scaffold by Fusion Peptides-Mediated Small Extracellular Vesicles of Stem Cells for Bone Tissue Regeneration. ACS Appl Mater Interfaces. 2024; 16(3):3064-3081. [61] KLINGER A, KAWATA M, VILLALOBOS M, et al. Living scaffolds: surgical repair using scaffolds seeded with human adipose-derived stem cells. Hernia. 2016;20:161-170. [62] WANG S, YAN H, FANG B, et al. A myogenic niche with a proper mechanical stress environment improves abdominal wall muscle repair by modulating immunity and preventing fibrosis. Biomaterials. 2022; 285:121519. [63] PREVEL CD, EPPLEY BL, SUMMERLIN DJ, et al. Small intestinal submucosa: utilization for repair of rodent abdominal wall defects. Ann Plast Surg. 1995;35(4):374-380. [64] CAO G, HE W, FAN Y, et al. Exploring the match between the degradation of the ECM-based composites and tissue remodeling in a full-thickness abdominal wall defect model. Biomater Sci. 2021;9(23): 7895-7910. [65] LI B, ZHANG X, MAN Y, et al. Lichtenstein inguinal hernia repairs with porcine small intestine submucosa: a 5-year follow-up. a prospective randomized controlled study. Regener Biomater. 2021;8(1):rbaa055. [66] JAMES N, POOLE-WARREN L, SCHINDHELM K, et al. Comparative evaluation of treated bovine pericardium as a xenograft for hernia repair. Biomaterials. 1991;12(9):801-809. [67] RENNEKAMPFF HO, DOHRMANN P, FÖRY R, et al. Evaluation of amniotic membrane as adhesion prophylaxis in a novel surgical gastroschisis model. J Invest Surg. 1994;7(3):187-193. [68] SZABO A, HAJ M, WAXSMAN I, et al. Evaluation of seprafilm and amniotic membrane as adhesion prophylaxis in mesh repair of abdominal wall hernia in rats. Eur Surg Res. 2000;32(2):125-128. [69] DYDAK K, JUNKA A, NOWACKI G, et al. In vitro cytotoxicity, colonisation by fibroblasts and antimicrobial properties of surgical meshes coated with bacterial cellulose. Int J Mol Sci. 2022;23(9):4835. [70] GULMEZ M, AKTEKIN A, AKER F, et al. Evaluation of In Vivo Adhesion Properties of New Generation Polyglactin, Oxidized Regenerated Cellulose and Chitosan-Based Meshes for Hernia Surgery. Cureus. 2021; 13(10):e18755. [71] HOSSIN MA, AL SHAQSI NHK, AL TOUBY SSJ, et al. A review of polymeric chitin extraction, characterization, and applications. Arabian J Geosci. 2021;14(18):1870. [72] YIN X, HAO Y, LU Y, et al. Bio‐multifunctional hydrogel patches for repairing full‐thickness abdominal wall defects. Adv Funct Mater. 2021; 31(41):2105614. [73] ZHANG W, LI Y, JIANG D, et al. Promotion of hernia repair with high-strength, flexible, and bioresorbable silk fibroin mesh in a large abdominal hernia model. ACS Biomater SciEng. 2017;4(6):2067-2080. [74] HE K, WU Z, WEN J, et al. Silk Fibroin Scaffolds Facilitating the Repair of Rat Abdominal Wall Defect. J Biomater Tissue Eng. 2016;6(8):665-671. [75] CLEMENS MW, DOWNEY S, AGULLO F, et al. Clinical application of a silk fibroin protein biologic scaffold for abdominal wall fascial reinforcement. Plast Reconstr Surg Glob Open. 2014;2(11):e246. [76] JANANI G, ZHANG L, BADYLAK S F, et al. Silk fibroin bioscaffold from Bombyx mori and Antheraea assamensis elicits a distinct host response and macrophage activation paradigm in vivo and in vitro. Biomater Adv. 2023;145:213223. [77] SORUSHANOVA A, DELGADO LM, WU Z, et al. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv Mater. 2019;31(1):1801651. [78] LI H, YOU S, YANG X, et al. Injectable recombinant human collagen-derived material with high cell adhesion activity limits adverse remodelling and improves pelvic floor function in pelvic floor dysfunction rats. Biomater Adv. 2022;134:112715. [79] QU L, CHEN Z, CHEN J, et al. Collagen biomaterials promote the regenerative repair of abdominal wall defects in Bama miniature pigs. Biomater Sci. 2023;11(24):7926-7937. [80] LIU S, ZHANG H, HU Q, et al. A facile strategy for fabricating composite patch scaffold by using porcine acellular dermal matrix and gelatin for the reconstruction of abdominal wall defects. J Biomater Appl. 2020;34(10):1479-1493. [81] BELLO AB, KIM D, KIM D, et al. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng Part B. 2020;26(2):164-180. [82] SERAFIM A, CECOLTAN S, OLĂREȚ E, et al. Bioinspired Hydrogel Coating Based on Methacryloyl Gelatin Bioactivates Polypropylene Meshes for Abdominal Wall Repair. Polymers (Basel). 2020;12(8):1677. [83] FERRANDO JM, VIDAL J, ARMENGOL M, et al. Experimental evaluation of a new layered prosthesis exhibiting a low tensile modulus of elasticity: long-term integration response within the rat abdominal wall. World J Surg. 2002;26:409-415. [84] QIAO Y, LI Y, ZHANG Q, et al. Dopamine-Mediated Zwitterionic Polyelectrolyte-Coated Polypropylene Hernia Mesh with Synergistic Anti-inflammation Effects. Langmuir. 2020;36(19):5251-5261. [85] HU Q, WU J, ZHANG H, et al. Designing Double-Layer Multimaterial Composite Patch Scaffold with Adhesion Resistance for Hernia Repair. Macromol Biosci. 2022;22(6):e2100510. [86] LUAN F, CAO W, CAO C, et al. Construction and properties of the silk fibroin and polypropylene composite biological mesh for abdominal incisional hernia repair. Front Biogeng Biotech. 2022;10:949917. [87] RONG L, YANG D, WANG B, et al. Durable and Effective Antibacterial Cotton Fabric Collaborated with Polypropylene Tissue Mesh for Abdominal Wall Defect Repair. ACS Biomater Sci Eng. 2020;6(7): 3868-3877. [88] ZHANG N, HUANG Y, WEI P, et al. Killing two birds with one stone: A therapeutic copper-loaded bio-patch promoted abdominal wall repair via VEGF pathway. Mater Today Bio. 2023;22:100785. [89] ZHANG M, YANG Y, LI M, et al. Toughening Double‐Network Hydrogels by Polyelectrolytes. Adv Mater. 2023;35(26):e2301551. [90] WILDY M, WEI W, XU K, et al. Exploring temperature-responsive drug delivery with biocompatible fatty acids as phase change materials in ethyl cellulose nanofibers. Int J Biol Macromol. 2024;266(Pt 1):131187. |
| [1] | Liu Zhongyu, Li Wenya, Fan Yonghong, Lyu Shuang, Pei Juan, Chen Yaqin, Liu Beiyu, Sun Hongyu. Methacrylated dermal extracellular matrix hydrogel promotes repair of abdominal wall defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2074-2082. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||