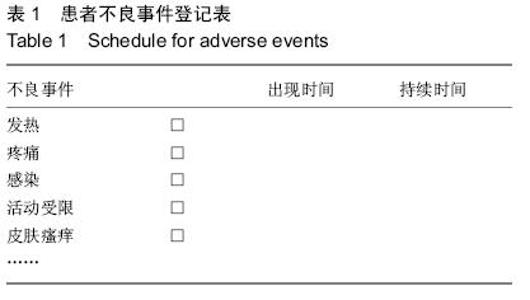
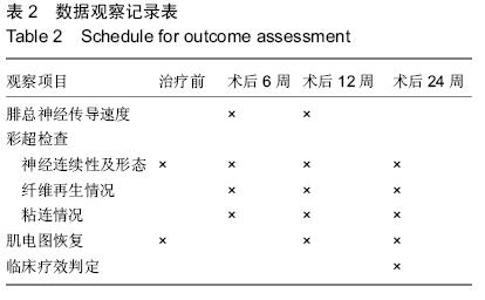
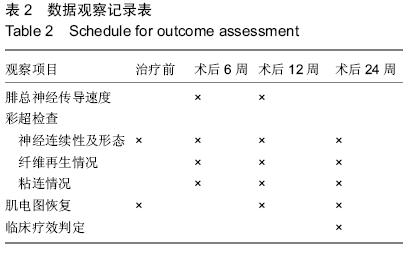
| [1] 李培建,李兵仓.周围神经损伤的修复及基因治疗[J].创伤外科杂志,.2002,4(l):59-61.[2] Noble F, Wank SA, Crawley JN,et al.International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors.Pharmacol Rev. 1999;51(4):745-781.[3] D'Angelo L, Castaldo L, Cellerino A, et al.Nerve growth factor in the adult brain of a teleostean model for aging research: Nothobranchius furzeri.Ann Anat. 2014; 196(4):183-191.[4] Sofroniew MV, Howe CL, Mobley WC.Nerve growth factor signaling, neuroprotection, and neural repair.Annu Rev Neurosci. 2001;24:1217-1281.[5] 路青林,刘庆胜,李志,等.神经节苷脂神经生长因子促进周围神经再生的实验研究[J].生物骨科材料与临床研究, 2007,4(2):40-42.[6] D'Angelo L, Castaldo L, Cellerino A, et al.Nerve growth factor in the adult brain of a teleostean model for aging research: Nothobranchius furzeri.Ann Anat. 2014; 196(4):183-191. [7] Sun XJ, Lu QC, Cai Y.Effect of cholecystokinin on experimental neuronal aging.World J Gastroenterol. 2005;11(4):551-556 [8] Katsuura G, Shinohara S, Shintaku H, et al.Protective effect of CCK-8 and ceruletide on glutamate-induced neuronal cell death in rat neuron cultures: possible involvement of CCK-B receptors.Neurosci Lett. 1991; 132(2):159-162.[9] Eigyo M, Katsuura G, Shintaku H,et al.Systemic administration of a cholecystokinin analogue, ceruletide, protects against ischemia-induced neurodegeneration in gerbils.Eur J Pharmacol. 1992; 214(2-3):149-158.[10] 梁卫兰,熊晖,母得志,等.八肽胆囊收缩素对神经细胞缺氧的作用[J].中华围产医学杂志,2000,3(2):120-123.[11] 杨世方,凌亦凌,凌毅群,等.中枢八肽胆囊收缩素对大鼠局部脑缺血/再灌注损伤的影响[J].中国病理生理杂志, 2003, 19(4):472-476.[12] 李德生,许百男,王世波,等.八肽胆囊收缩素抑制大鼠脊髓损伤后诱导型一氧化氮合酶的表达[J].中国临床康复, 2005,9(9):24-27.[13] 韦鹏,凌亦凌,牛志云,等.八肽胆囊收缩素对内毒素休克时大脑皮质损伤的影响及其机制初探[J].河北医科大学学报,2004,25(4):225-226.[14] Tirassa P, Stenfors C, Lundeberg T,et al. Cholecystokinin-8 regulation of NGF concentrations in adult mouse brain through a mechanism involving CCK(A) and CCK(B) receptors.Br J Pharmacol. 1998; 123(6):1230-1236.[15] Xanthos DN, Kumar N, Theodorsson E,et al.The roles of nerve growth factor and cholecystokinin in the enhancement of morphine analgesia in a rodent model of central nervous system inflammation. Neuropharmacology. 2009; 56(3):684-691.[16] Manni L, Lundeberg T, Tirassa P,et al. Cholecystokinin-8 enhances nerve growth factor synthesis and promotes recovery of capsaicin-induced sensory deficit.Br J Pharmacol. 2000;129(4):744-750.[17] 陈宣煌,李荣议,张国栋,等.胆囊收缩素促坐骨神经再生的可能机制[J].中国组织工程研究,2014,18(11):1700-1705.[18] 陈宣煌,林海滨,吴献伟,等.胆囊收缩素促大鼠坐骨神经再生的初步研究[J/CD].中华临床医师杂志:电子版, 2011, 5(24):7242-7247.[19] 陈宣煌,郑祖高,占鲤生,等.胆囊收缩素对大鼠坐骨神经损伤后修复的影响[J].南昌大学学报(医学版),2012,52(7): 4-7.[20] Tirassa P, Manni L, Aloe L, et al.Cholecystokinin-8 and nerve growth factor: two endogenous molecules working for the upkeep and repair of the nervous system.Curr Drug Targets CNS Neurol Disord. 2002; 1(5):495-510.[21] Tirassa P, Costa N.CCK-8 induces NGF and BDNF synthesis and modulates TrkA and TrkB expression in the rat hippocampus and septum: Effects on kindling development.Neurochem Int. 2007; 50(1):130-138. |