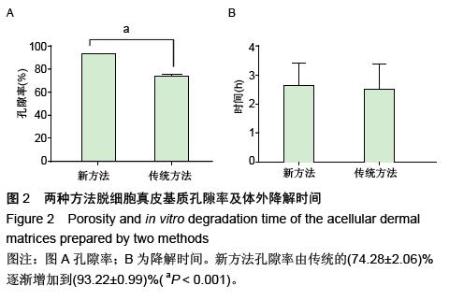
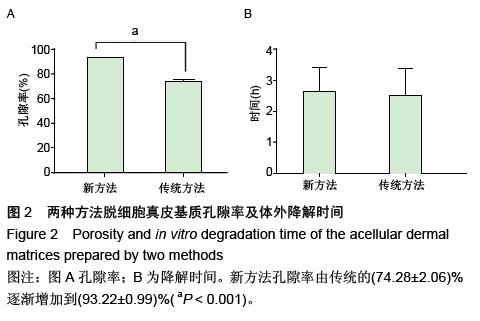
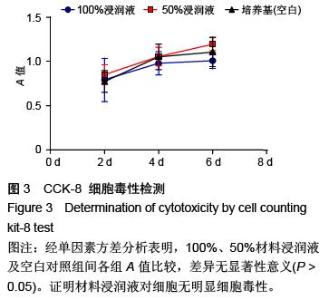
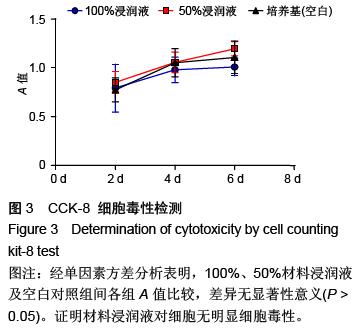
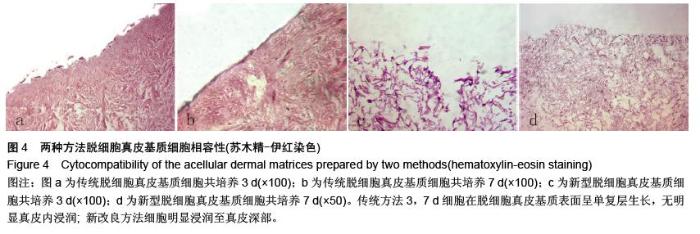
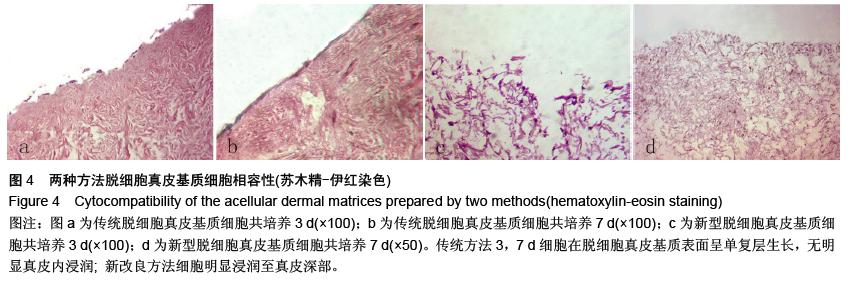
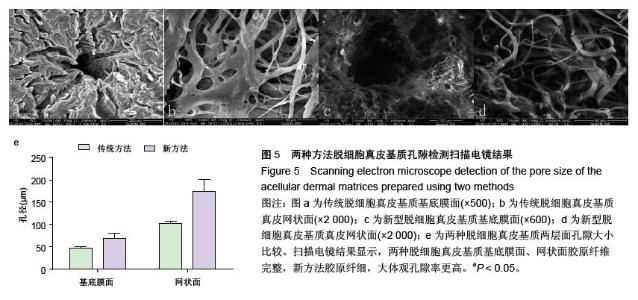
| [1] Hart CE, Loewen-Rodriguez A, Lessem J. Dermagraft: Use in the Treatment of Chronic Wounds. Advances in wound care.2012;1(3):138-141.
[2] Huang SP, Hsu CC, Chang SC, et al. Adipose-derived stem cells seeded on acellular dermal matrix grafts enhance wound healing in a murine model of a full-thickness defect. Ann Plast Surg. 2012;69(6): 656-662.
[3] Nie C, Yang D, Morris SF. Local delivery of adipose- derived stem cells via acellular dermal matrix as a scaffold: a new promising strategy to accelerate wound healing. Med Hypotheses.2009;72(6): 679-682.
[4] Kirsner RS, Bohn G, Driver VR, et al. Human acellular dermal wound matrix: evidence and experience. Int Wound J. 2015;12(6):646-654
[5] Debels H, Hamdi M, Abberton K, et al. Dermal matrices and bioengineered skin substitutes: a critical review of current options. Plast Reconstr Surg Glob Open. 2015; 3(1): e284.
[6] 张爱君,宋国栋,曹成波,等.猪胶原基真皮支架成纤维细胞生长特性的观察[J].山东大学学报:医学版,2007,45(7): 718-21,25.
[7] Xu H, Sandor M, Qi S, et al. Implantation of a porcine acellular dermal graft in a primate model of rotator cuff repair. J Shoulder Elbow Surg. 2012;21(5):580-588.
[8] Zhang X, Deng Z, Wang H, et al.Expansion and delivery of human fibroblasts on micronized acellular dermal matrix for skin regeneration. Biomaterials. 2009;30(14): 2666-2674.
[9] 冯祥生,潘银根,谭家驹.异种(猪)脱细胞真皮与自体表皮复合移植研究[J].中华整形外科杂志, 2000,16(1): 40.
[10] Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell.2002;13(12): 4279-4295.
[11] 刘静,李强,陶常波,等.人脂肪间充质干细胞膜片的生物学性状研究[J].徐州医学院学报, 2015, 35(3): 181-184.
[12] 郭艳萍,张爱君,马志兵等.人体脂肪干细胞培养及成骨诱导分化[J].徐州医学院学报,2014, 34(1): 22-26.
[13] Hansbrough JF, Cooper ML, Cohen R, et al. Evaluation of a biodegradable matrix containing cultured human fibroblasts as a dermal replacement beneath meshed skin grafts on athymic mice. Surgery.1992;111(4): 438-446.
[14] Panuncialman J, Falanga V. The science of wound bed preparation. Clinics in plastic surgery.2007;34(4): 621-632.
[15] 张爱君,李强,崔莹莹,等.不同年龄阶段人脱细胞真皮的制备及细胞渗透性研究[J].中华医学美学美容杂志, 2014, 20(2): 130-3.
[16] Song G, Wu Y, Wang F, et al. Development and preparation of a low-immunogenicity porcine dermal scaffold and its biocompatibility assessment. J Mater Sci Mater Med. 2015;26(4):170.
[17] 张爱君.新型猪胶原基真皮支架细胞毒性及与细胞相容性的实验研究[D].山东大学,2007.
[18] 肖和兰,孙素琴,周群,等.温度对胶原蛋白结构影响的二维红外相关光谱的研究[J].原子与分子物理学报, 2003, 20(2): 211-218.
[19] Sun Y, Chen WL, Lin SJ, et al. Investigating mechanisms of collagen thermal denaturation by high resolution second-harmonic generation imaging. Biophys J. 2006 1;91(7):2620-2625.
[20] 李文波,胡顺鹏,赵洪石,等.脱细胞真皮基质微结构的碱法调控及其细胞相容性[J].化工学报, 2011, 62(2): 545-50.
[21] Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng.2001;7(2): 211-228.
[22] Yang XF, He X, He J, et al. High efficient isolation and systematic identification of human adipose-derived mesenchymal stem cells. J Biomedical Sci.2011;18:59.
[23] Li Q, Li PH, Hou DJ, et al. EGF enhances ADSCs secretion via ERK and JNK pathways. Cell Biochem Biophys. 2014;69(1):189-196.
[24] Li Q, Zhang A, Tao C, et al. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem Biophys Res Commun. 2013;441(3): 675-680.
[25] 李强,金培生,张爱君等.脂肪干细胞原代培养及其向表皮细胞诱导分化的研究[J].中华医学美学美容杂志, 2012, 18(6): 440,443.
[26] Scherzed A, Hackenberg S, Froelich K, et al. The effect of wound fluid on adipose-derived stem cells in vitro: a study in human cell materials. Tissue engineering Part C, Methods. 2011;17(8): 809-817.
[27] Meruane MA, Rojas M, Marcelain K. The use of adipose tissue-derived stem cells within a dermal substitute improves skin regeneration by increasing neoangiogenesis and collagen synthesis. Plast Reconstr Surg. 2012;130(1):53-63.
[28] Iyyanki TS, Dunne LW, Zhang Q, et al. Adipose-derived stem-cell-seeded non-cross-linked porcine acellular dermal matrix increases cellular infiltration, vascular infiltration, and mechanical strength of ventral hernia repairs. Tissue engineering Part A. 2015;21(3-4): 475-485.
[29] Chang P, Tao K.The use of adipose tissue-derived stem cells within a dermal substitute improves skin regeneration by increasing neoangiogenesis and collagen synthesis. Plast Reconstr Surg. 2013;131(1): 116e-7e.
[30] Zamora DO, Natesan S, Becerra S, et al. Enhanced wound vascularization using a dsASCs seeded FPEG scaffold. Angiogenesis.2013;16(4): 745-757. |