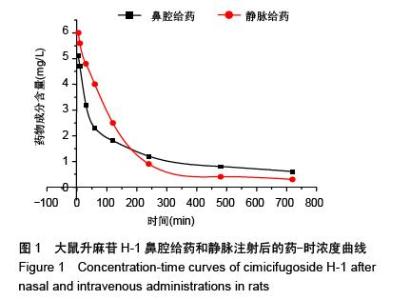
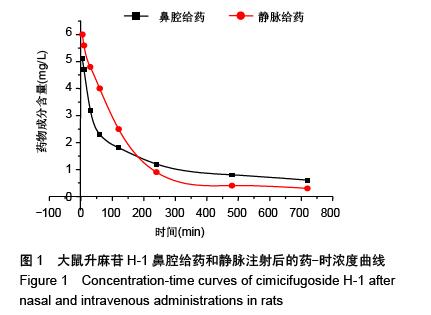
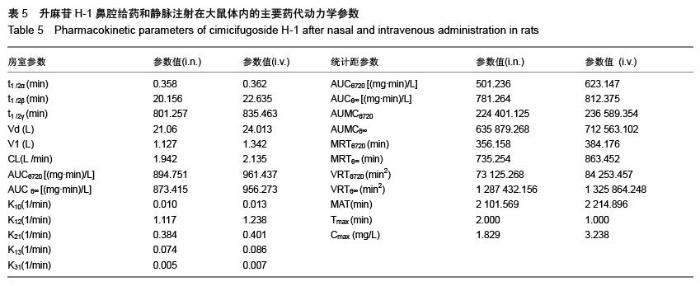
| [1] 张仲明,江波.脑缺血治疗药物的研究进展[J].中国现代应用药学杂志,2006,23(1):24-27.
[2] 吴德松,卿晨.升麻药理学活性研究进展[J].医学综述, 2009, 15(6): 918-920.
[3] 林玉萍,邱明华,李忠荣. 升麻属植物的化学成分与生物活性研究[J].天然产物研究与开发,2002,14(6):58-68.
[4] 刘颖,张小茜.升麻质量标准的研究[J].中草药,2005,36(9): 1402-1404.
[5] 潘瑞乐,陈迪华,沈连钢.高效液相色谱法测定中药升麻中阿魏酸和异阿魏酸的含量[J].药物分析杂志,2000,20(6):396-398.
[6] 姚梅芬,王岳峰,李展,等.反相高效液相色谱法测定升麻药材中升麻苷H-1 的含量[J].天然产物研究与开发,2011,23(4):696-699.
[7] 杨莉,高颖昌,赵志刚.鼻腔给药的研究进展[J].中国药学杂志, 2006,41(22):1685-1688.
[8] Koeda M, Aoki Y, Sakurai N, et al. Studies on the Chinese crude drug”shoma.” IX. Three novel cyclolanostanol xylosides, cimicifugosides H-1, H-2 and H-5, from cimicifuga rhizome. Chem Pharm Bull (Tokyo). 1995;43(5):771-776.
[9] Yajima T, Juni K, Saneyoshi M, et al. Direct transport of 2’,3’-didehydro-3’-deoxythymidine (D4T) and its ester derivatives to the cerebrospinal fluid via the nasal mucous membrane in rats.Biol Pharm Bull. 1998;21(3):272-277.
[10] Illum L.Nasal drug delivery: new developments and strategies.Drug Discov Today. 2002;7(23):1184-1189.
[11] Illum L.Is nose-to-brain transport of drugs in man a reality. J Pharm Pharmacol. 2004;56(1):3-17.
[12] Graff CL, Pollack GM.Nasal drug administration: potential for targeted central nervous system delivery.J Pharm Sci. 2005; 94(6):1187-1195.
[13] Illum L.Nasal drug delivery--possibilities, problems and solutions.J Control Release. 2003;87(1-3):187-198.
[14] Dhuria SV, Hanson LR, Frey WH 2nd.Intranasal delivery to the central nervous system: mechanisms and experimental considerations.J Pharm Sci. 2010;99(4):1654-1673.
[15] Thorne RG, Pronk GJ, Padmanabhan V, et al. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration.Neuroscience. 2004;127(2):481-496.
[16] Illum L.Nasal drug delivery--possibilities, problems and solutions.J Control Release. 2003;87(1-3):187-198.
[17] Evans J, Hastings L.Accumulation of Cd(II) in the CNS depending on the route of administration: intraperitoneal, intratracheal, or intranasal.Fundam Appl Toxicol. 1992;19(2): 275-278. |