Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (49): 8602-8607.doi: 10.3969/j.issn.2095-4344.2013.49.022
Previous Articles Next Articles
Autologous stem cells transplantation in the treatment of critical limb ischemia
Li Mao, Huang Wen
- First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China
-
Revised:2013-09-02Online:2013-12-03Published:2013-12-03 -
Contact:Huang Wen, M.D., Chief physician, First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China dhuangwen@hotmail.com -
About author:Li Mao★, Studying for master’s degree, Attending physician, First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China limao19750204@126.com -
Supported by:the General Program of National Natural Science Foundation of China, No. 81070255*
CLC Number:
Cite this article
Li Mao, Huang Wen. Autologous stem cells transplantation in the treatment of critical limb ischemia[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(49): 8602-8607.
share this article

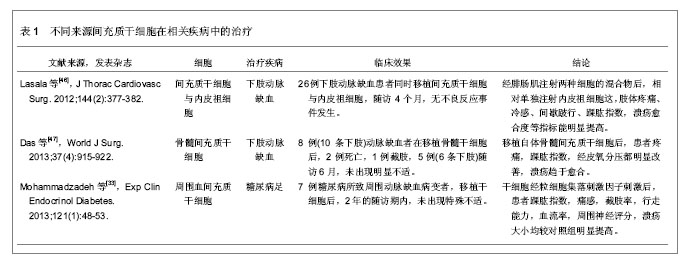
2.1 干细胞的分类 干细胞是一种可自我更新、增殖和多向分化潜能的细胞。干细胞可分为胚胎干细胞和成体干细胞。胚胎干细胞由于需要杀死胚胎,违背传统伦理学观点,在临床应用中受到限制,因而成体干细胞移植具有广泛的临床应用前景。 2.2 成体干细胞的来源和功能 研究发现成体干细胞存在于很多器官中, 包括脑、骨髓、外周血、血管组织、肌肉、牙齿、肝脏和皮肤等组织和器官,该类细胞的主要功能是组织修复,维持如血液、皮肤和肠道等组织的更新。干细胞一般位于组织或器官的某一特殊部位(干细胞巢),例如在小血管的最外层存在一种血管干细胞叫周细。多数成体干细胞例如间充质干细胞、脂肪干细胞和内皮祖细胞等为多能干细胞,多能干细胞可分化为多种细胞,但是不能跨系分化,仅可以分化为同系的成熟细胞[9]。骨髓源干细胞被证实为一潜在干细胞来源。研究发现骨髓包含两类干细胞:造血干细胞和骨髓间充质干细胞。造血干细胞形成各类血细胞。间充质干细胞在骨髓中比例较少,但具有分化为骨、软骨、脂肪等细胞的潜能,同时协助生成血细胞和纤维结缔组织的功能。1997年Isner等[10]最早在外周血分离出内皮祖细胞,发现该细胞具有分化为血管内皮细胞的功能,并可整合入缺血组织所形成的新生血管内,随后大量报道证实内皮祖细胞与血管新生的关系[11]。 血管新生有3种方式:血管形成即血管通过发芽的方式新生血管,血管发生即胚胎时期血管组织的发育,动脉生成即侧支循环建立。目前临床应用的自体骨髓间充质干细胞移植治疗周围血管闭塞性疾病就是模仿血管形成的一种治疗方式,但目前其机制尚不清楚。Tateishi-Yuyama等[12]首次报道移植骨髓自体单个核细胞治疗下肢缺血性疾病,患者移植单个核细胞后患肢皮温升高,踝肱指数明显改善。Yang等[13]也报道粒细胞集落刺激因子动员骨髓后移植外周血单个核细胞取得较好疗效。Li等[14]运用骨髓间充质干细胞治疗下肢缺血疾病时,对于静息痛和皮温等临床表现有显著改善。Subrammaniyan等[15]和Murphy等[16]通过临床试验证实骨髓间充质干细胞治疗严重肢体缺血患者时,其安全性和疗效都得到证实。但Jonsson在评价骨髓间充质干细胞治疗严重肢体缺血的疗效时,发现经干细胞治疗后可能发生多种不良事件[17]。因此,对于骨髓间充质干细胞治疗严重肢体缺血的疗效尚需更多的临床资料进行评估,还需优化干细胞移植治疗方法。 不同研究移植的干细胞亚群不同,有研究者采集外周血单个核细胞或采集CD34+细胞,或采集CD133+细胞。还有研究者在体外培养外周血单个核细胞后进行移植。由于没有不同细胞亚群的临床疗效的直接比较,因此何种干细胞亚群的疗效更好尚不清楚[18]。有研究显示CD34+细胞与血管修复以及血管新生有明显关系[19],外周血单个核细胞的CD34+细胞含量较骨髓间充质干细胞的低500倍,外周血单个核细胞移植后的下肢侧枝循环建立较骨髓间充质干细胞移植后的差[20]。Saigawa等[21]也证实移植不同部位的CD34+细胞和疗效有密切关系。但是很多研究显示CD34-细胞在骨髓间充质干细胞移植中也起重要作用,Asahara等[11]报道CD34-细胞与CD34+细胞共培养,可以提高干细胞的内皮细胞表型的表达。CD34+细胞和CD34-细胞与人微血管内皮细胞在体外3D基质中共培养,较单纯CD34+细胞与微血管内皮细胞共培养在移植后其新生血管更明显[20,22]。其他研究显示非造血骨髓间充质干细胞前体细胞和骨髓单核细胞(myeloid/monocyte lineage cells)也可分化为内皮细胞和内皮细胞样细胞[23]。Iba等[24]比较相同细胞数的骨髓间充质干细胞和外周血单个核细胞(分别含有2.4%和0.02% CD34+细胞)分别移植后在大鼠后腿缺血模型的成血管效应,外周血单个核细胞成血管效应仅达到骨髓间充质干细胞的72%[25]。但是 Tateno等[10]却报道外周血单个核细胞和骨髓间充质干细胞刺激血管形成效应没有区别。 内皮祖细胞除具有形成血管的功能外,也分泌血管内皮生长因子、碱性成纤维细胞生长因子和血管生成素1[26-27]。Yang等[28]也证实了内皮祖细胞具有刺激血管新生的旁分泌作用。Tsukada等[29]用内皮祖细胞治疗大鼠缺血模型后,发现新生血管明显多于阴性对照组。最近研究报道干细胞移植刺激肌肉细胞产生血管生成因子从而诱导血管形成[10]。因此间充质干细胞移植治疗严重肢体缺血的机制也许是干细胞直接融合入血管进而分化为内皮细胞,或仅具有旁分泌功能,或同时具有以上两种作用[30]。其中机制需要更多的深入研究。 2.3 影响干细胞移植疗效的因素 2.3.1 自体单个核细胞获取方法对疗效的影响 虽然临床研究发现骨髓间充质干细胞和粒细胞集落刺激因子动员的周血单个核细胞有较好疗效,但两者在疗效的比较中并没有差异[31]。Dubsky等[32]用粒细胞集落刺激因子动员后的周血单个核细胞治疗2型糖尿病并发的严重肢体缺血,证实干细胞经粒细胞集落刺激因子动员后治疗严重肢体缺血更具有安全性和有效性。Mohammadzadeh等[33]用干细胞移植治疗糖尿病足合并严重肢体缺血时,也发现粒细胞集落刺激因子动员后的周血单个核细胞移植者,新生血管更丰富,溃疡更容易愈合。动物实验发现裸鼠移植经过粒细胞集落刺激因子动员的内皮祖细胞的疗效,较移植未经粒细胞集落刺激因子动员的内皮祖细胞的疗效差[34]。有报道细胞分离技术也是影响疗效的重要原因[35]。 2.3.2 移植的细胞数量对疗效的影响 虽然所有研究均报道取得良好效果,但是不同的研究所移植的单个核细胞数量却明显不同,CD34+细胞数量也不一致。在骨髓单个核细胞移植的报道中,抽取的骨髓量介于80-1 000 mL。Tateishi-Yuyama等[12]报道在500 mL骨髓里可获取约1.6×109个单个核细胞,而Yoshitaka等[36]则报道在500 mL骨髓内提取了34×109个单个核细胞,其数量是上述研究的20倍。而Bartsch等[35]从 80 mL骨髓内分离出的干细胞数量却仅为0.1×109单个核细胞。分离的单个核细胞细胞液中 CD34+细胞比例也变化于0.6%-2.4%[18]。 2.3.3 其他因素 自体单个核细胞移植者的年龄,注射部位,病程长短,是否合并并发症,安全性和稳定性等都是影响因素。Iso等[36]用骨髓间充质干细胞治疗慢性严重肢体缺血后,其恢复效果明显慢于急性发病者。Klepanec等[37]将自体干细胞注射在血管内和肌肉内治疗严重肢体缺血时,发现两种注射方式对疗效并无差异。老龄患者接受干细胞治疗后的恢复时间和效果都明显不如年龄较小的患者。Keller[38]对以往文献进行综述时,分离于骨髓间充质干细胞的醛脱氢酶(ldehyde dehydrogenase)对严重肢体缺血的疗效较单纯注射骨髓间充质干细胞的更具安全性和疗效稳定性。Perin等[39]通过临床随机对照研究证实了这种说法。 2.4 疗效评估方法 目前评价下肢血供的手段比较局限,最常用的是踝肱指数等指标,但这些指标对下肢的组织灌注反映较少。Choksy和Chan指出自体单个核细胞移植疗效缺乏客观的评估手段是自体单个核细胞移植治疗严重肢体缺血研究的主要缺陷[40]。根据泛大西洋协作组织(TASC-II)对下肢缺血性疾病的治疗建议,自体单个核细胞移植的疗效评估需要包括如下等多个方面:临床症状改善、组织血流灌注变化(踝肱指数、足趾血压、透皮氧分压、激光多普勒血流检测)、生物活性因子的表达,影像学检查以及注射方式等[41-44]。问卷调查需要包括疼痛时间、疼痛程度(如疼痛量表)等,同时也需问及生活质量,需要建立以患者为本的疗效评估体系[1]。溃疡愈合需描述累计溃疡面积, 溃疡愈合定义为下肢所有溃疡愈合。下肢缺血状况评估可采用 Rutherford分级。高分辨率增强MRI是一可重复和无创的新生血管检测手段,可检测侧枝循环建立程度和组织灌注情况[45]。 2.5 相关细胞在不同疾病的治疗及效果 见表1。"
| [1] Norgren L, Hiatt WR, Dormandy JA,et al. Inter-society consensus for the management of peripheral arterial disease(TASC II). Eur J Vasc Endovasc Surg.2007;33(Suppl 1):S1-S75.[2] Casserly IP. Interventional management of critical limb ischemia in renal patients. Adv Chronic Kidney Dis. 2008; 15(4):384-395.[3] Ubbink DT, Vermeulen H. Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev. 2013;2:CD004001.[4] Dohmen A, Eder S, Euringer W, et al. Chronic critical limb ischemia. Dtsch Arztebl Int. 2012;109(6):95-101. [5] Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239-1312. [6] Hooi JD, Kester AD, Stoffers HE, et al. Asymptomatic peripheral arterial occlusive disease predicted cardiovascular morbidity and mortality in a 7-year follow-up study. J Clin Epidemiol. 2004;57(3):294-300.[7] Tateno K, Minamino T, Toko H, et al. Critical roles of muscle-secreted angiogenic factors in therapeutic neovascularization. Circ Res. 2006;98(9):1194-1202. [8] Collinson DJ, Donnelly R. Therapeutic angiogenesis in peripheral arterial disease: can biotechnology produce an effective collateral circulation? Eur J Vasc Endovasc Surg. 2004;28(1):9-23.[9] Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249-1260. [10] Barrilleaux B, Phinney DG, Prockop DJ, et al. Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12(11):3007-3019. [11] Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275(5302):964-967.[12] Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360(9331): 427-435. [13] Ruifrok WP, de Boer RA, Iwakura A, et al. Estradiol-induced, endothelial progenitor cell-mediated neovascularization in male mice with hind-limb ischemia. Vasc Med. 2009;14(1): 29-36. [14] Li M, Zhou H, Jin X, et al. Autologous Bone Marrow Mononuclear Cells Transplant in Patients With Critical Leg Ischemia: Preliminary Clinical Results. Exp Clin Transplant. 2013 Mar 11.[15] Subrammaniyan R, Amalorpavanathan J, Shankar R, et al. Application of autologous bone marrow mononuclear cells in six patients with advanced chronic critical limb ischemia as a result of diabetes: our experience. Cytotherapy. 2011;13(8): 993-999.[16] Murphy MP, Lawson JH, Rapp BM, et al. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J Vasc Surg. 2011;53(6):1565-1574.[17] Jonsson TB, Larzon T, Arfvidsson B, et al. . Adverse events during treatment of critical limb ischemia with autologous peripheral blood mononuclear cell implant. Int Angiol .2012; 31(1):77-84.[18] Lin MN, Shang DS, Sun W, et al. Involvement of PI3K and ROCK signaling pathways in migration of bone marrow-derived mesenchymal stem cells through human brain microvascular endothelial cell monolayers. Brain Res. 2013;1513:1-8.[19] Saigawa T, Kato K, Ozawa T, et al. Clinical application of bone marrow implantation in patients with arteriosclerosis obliterans, and the association between efficacy and the number of implanted bone marrow cells. Circ J. 2004;68(12): 1189-1193. [20] Rookmaaker MB, Verhaar MC, Loomans CJ, et al. CD34+ cells home, proliferate, and participate in capillary formation, and in combination with CD34- cells enhance tube formation in a 3-dimensional matrix. Arterioscler Thromb Vasc Biol. 2005; 25(9):1843-1850. [21] Losordo DW, Kibbe MR, Mendelsohn F, et al. Autologous CD34+ Cell Therapy for Critical Limb Ischemia Investigators. Circ Cardiovasc Interv. 2012;5(6):821-830.[22] Stec M, Baran J, Szatanek R, et al. Interactions of monocyte subpopulations generated from cord blood CD34(+) hematopoietic progenitors with tumor cells: assessment of antitumor potential. Exp Hematol. 2012;40(11):914-921. [23] Fernandez Pujol B, Lucibello FC, Gehling UM, et al. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000;65(5):287-300. [24] Iba O, Matsubara H, Nozawa Y, et al. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106(15): 2019-2025. [25] Zhang YY, Yue J, Che H, et al. BKCa and hEag1 channels regulate cell proliferation and differentiation in human bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2013 Jul 23.[26] Heil M, Ziegelhoeffer T, Mees B, et al. A different outlook on the role of bone marrow stem cells in vascular growth: bone marrow delivers software not hardware. Circ Res. 2004;94(5): 573-574. [27] Shen WC, Liang CJ, Wu VC, et al.Endothelial progenitor cells derived from Wharton's jelly of the umbilical cord reduces ischemia-induced hind limb injury in diabetic mice by inducing HIF-1α/IL-8 expression.Stem Cells Dev. 2013;22(9):1408- 1418. [28] Yang XF, Wu YX, Wang HM, et al. Autologous peripheral blood stem cells transplantation in treatment of 62 cases of lower extremity ischemic disorder. Zhonghua Nei Ke Za Zhi. 2005;44(2):95-98. [29] Tsukada S, Kwon SM, Matsuda T, et al. Identification of mouse colony forming endothelial progenitor cells for postnatal neovascularization: a novel insight highlighted by new mouse colony forming assay. Stem Cell Res Ther. 2013; 4(1):20.[30] López M, San Román J, Estrada V, et al. Endothelial dysfunction in HIV infection--the role of circulating endothelial cells, microparticles, endothelial progenitor cells and macrophages. AIDS Rev. 2012;14(4):223-230. [31] Honold J, Lehmann R, Heeschen C, et al. Effects of granulocyte colony simulating factor on functional activities of endothelial progenitor cells in patients with chronic ischemic heart disease. Arterioscler Thromb Vasc Biol. 2006;26(10): 2238-2243. [32] Dubsky M, Jirkovska A, Bem R, et al. Both autologous bone marrow mononuclear cells and peripheral blood progenitor cells therapies similarly improve ischemia in patients with diabetic foot in comparison with control treatment.Diabetes Metab Res Rev. 2013 Feb 7.[33] Mohammadzadeh L, Samedanifard SH, Keshavarzi A, et al. Therapeutic outcomes of transplanting autologous granulocyte colony-stimulating factor-mobilised peripheral mononuclear cells in diabetic patients with critical limb ischaemia. Exp Clin Endocrinol Diabetes. 2013;121(1):48-53.[34] Seeger FH, Tonn T, Krzossok N, et al. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28(6):766-772. [35] Bartsch T, Falke T, Brehm M, et al. Intra-arterial and intramuscular transplantation of adult, autologous bone marrow stem cells. Novel treatment for therapy-refractory peripheral arterial occlusive disease. Dtsch Med Wochenschr. 2006;131(3):79-83. [36] Iso Y, Soda T, Sato T, et al. Impact of implanted bone marrow progenitor cell composition on limb salvage after cell implantation in patients with critical limb ischemia. Atherosclerosis. 2010;209(1):167-172.[37] Klepanec A, Mistrik M, Altaner C, et al. No difference in intra-arterial and intramuscular delivery of autologous bone marrow cells in patients with advanced critical limb ischemia. Cell Transplant. 2012;21(9):1909-1918.[38] Keller LH.Bone marrow-derived aldehyde dehydrogenase-bright stem and progenitor cells for ischemic repair. Congest Heart Fail. 2009;15(4):202-206.[39] Perin EC, Silva G, Gahremanpour A, et al. A randomized, controlled study of autologous therapy with bone marrow-derived aldehyde dehydrogenase bright cells in patients with critical limb ischemia.Catheter Cardiovasc Interv. 2011;78(7):1060-1067.[40] Wang P, Luo Y, Duan H, et al. MicroRNA-329 suppresses angiogenesis by targeting CD146. Mol Cell Biol. 2013.[41] Powell RJ, Comerota AJ, Berceli SA, et al. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. J Vasc Surg. 2011;54(4): 1032-1041.[42] Schiavetta A, Maione C, Botti C, et al.A phase II trial of autologous transplantation of bone marrow stem cells for critical limb ischemia: results of the Naples and Pietra Ligure Evaluation of Stem Cells study.Stem Cells Transl Med. 2012; 1(7):572-578.[43] Mamidi MK, Pal R, Dey S, et al.Cell therapy in critical limb ischemia: current developments and future progress. Cytotherapy. 2012;14(8):902-916.[44] Smadja DM, Duong-van-Huyen JP, Dal Cortivo L, et al.Early endothelial progenitor cells in bone marrow are a biomarker of cell therapy success in patients with critical limb ischemia. Cytotherapy. 2012;14(2):232-239. [45] Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517-538. [46] Lasala GP, Silva JA, Minguell JJ. Therapeutic angiogenesis in patients with severe limb ischemia by transplantation of a combination stem cell product. J Thorac Cardiovasc Surg. 2012;144(2):377-382. [47] Das AK, Bin Abdullah BJ, Dhillon SS, et al. Intra-arterial allogeneic mesenchymal stem cells for critical limb ischemia are safe and efficacious: report of a phase I study. World J Surg. 2013;37(4):915-922. |
| [1] | Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(在线): 3-. |
| [2] | Zhou Qi, Gao Yi, Wei Kang, Li Jun, Xu Jianda, Jiang Yang, Qu Yuxing. Total knee arthroplasty for rheumatoid arthritis: knee function and biochemical index changes [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(9): 1337-1341. |
| [3] | Chen Jinsong, Wang Zhonghan, Chang Fei, Liu He. Tissue engineering methods for repair of articular cartilage defect under special conditions [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(8): 1272-1279. |
| [4] | Zhang Shengmin, Liu Chao. Research progress in osteogenic differentiation of adipose-derived stem cells induced by bioscaffold materials [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1107-1116. |
| [5] |
Wang Tiantian, Wang Jianzhong.
Application and prospect of bone marrow mesenchymal stem cells in the
treatment of early femoral head necrosis |
| [6] | Wang Zhangling, Yu Limei, Zhao Chunhua. Tissue repair using mesenchymal stem cells via mitochondrial transfer [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1123-1129. |
| [7] | Deng Junhao, Li Miao, Zhang Licheng, Tang Peifu. Three-dimensional hanging-drop culture of mesenchymal stem cells in the treatment of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1101-1106. |
| [8] | Ren Chunmei, Liu Yufang, Xu Nuo, Shao Miaomiao, He Jianya, Li Xiaojie. Non-coding RNAs in human dental pulp stem cells: regulations and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1130-1137. |
| [9] | Huang Wenwen, Li Shuo, Hou Zongliu, Wang Wenju. Pathogenesis of inflammatory bowel disease and mesenchymal stem cell therapy: therapeutic application and existing problems [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1138-1143. |
| [10] | Liu Chundong, Shen Xiaoqing, Zhang Yanli, Zhang Xiaogen, Wu Buling. Effects of strontium-modified titanium surfaces on adhesion, migration and proliferation of bone marrow mesenchymal stem cells and expression of bone formation-related genes [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1009-1015. |
| [11] | Lin Ming, Pan Jinyong, Zhang Huirong. Knockout of NIPBL gene down-regulates the abilities of proliferation and osteogenic differentiation in mouse bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1002-1008. |
| [12] | Zhang Wen, Lei Kun, Gao Lei, Li Kuanxin. Neuronal differentiation of rat bone marrow mesenchymal stem cells via lentivirus-mediated bone morphogenetic protein 7 transfection [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 985-990. |
| [13] | Wu Zhifeng, Luo Min. Biomechanical analysis of chemical acellular nerve allograft combined with bone marrow mesenchymal stem cell transplantation for repairing sciatic nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 991-995. |
| [14] | Huang Yongming, Huang Qiming, Liu Yanjie, Wang Jun, Cao Zhenwu, Tian Zhenjiang, Chen Bojian, Mai Xiujun, Feng Enhui. Proliferation and apoptosis of chondrocytes co-cultured with TDP43 lentivirus transfected-human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1016-1022. |
| [15] | Qin Xinyu, Zhang Yan, Zhang Ningkun, Gao Lianru, Cheng Tao, Wang Ze, Tong Shanshan, Chen Yu. Elabela promotes differentiation of Wharton’s jelly-derived mesenchymal stem cells into cardiomyocyte-like cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1046-1051. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||