Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (35): 7656-7662.doi: 10.12307/2025.998
Previous Articles Next Articles
Effect of insulin-like growth factor family member levels on inflammatory arthritis: a FinnGen biobank-based analysis
Wang Xuepeng1, 2, He Yong1, 2
- 1Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; 2Shanghai Guanghua Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai 200052, China
-
Received:2024-11-07Accepted:2024-12-25Online:2025-12-18Published:2025-05-07 -
Contact:Corresponding author: He Yong, MD, Chief physician, Master’s supervisor, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; Shanghai Guanghua Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai 200052, China -
About author:Wang Xuepeng, Master, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; Shanghai Guanghua Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai 200052, China -
Supported by:“Science and Technology Innovation Action Plan” of Shanghai Municipal Science and Technology Commission for Medical Innovation Research Special Project, No. 21Y11911400 (to HY); Shanghai Changning District Medical Master and Doctoral Innovation Talent Base Project, No. RCJD2022S04 (to HY)
CLC Number:
Cite this article
Wang Xuepeng, , He Yong, . Effect of insulin-like growth factor family member levels on inflammatory arthritis: a FinnGen biobank-based analysis[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7656-7662.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
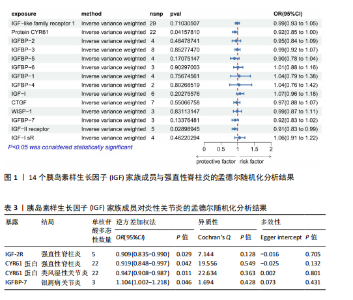
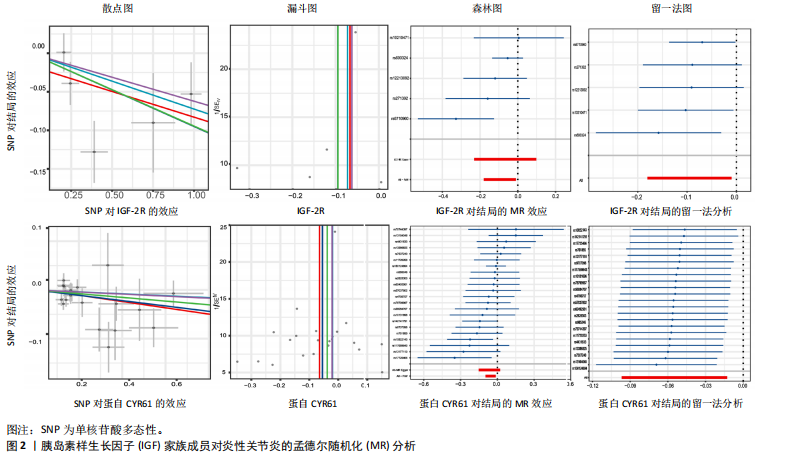
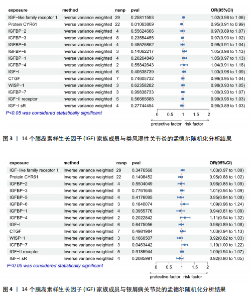
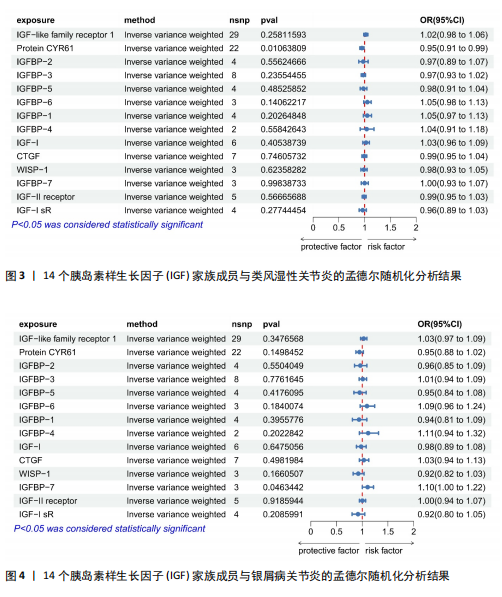
2.1 14个IGF家族成员对强直性脊柱炎的影响 在对工具变量进行严格的质量控制后,最终在孟德尔随机化研究中对14个IGF家族成员进行了分析。每个IGF家族成员的工具变量数量范围为2-29个。所有单核苷酸多态性的F统计量范围为19.6-450.8,均超过了阈值10,表明弱工具变量偏倚不太可能存在。 14个IGF家族成员与强直性脊柱炎风险之间关联的结果见图1。通过逆方差加权方法确定了2个IGF家族成员与强直性脊柱炎风险之间的潜在因果关联。在这2个IGF家族成员中,IGF-2R(OR:0.909,95%CI:0.835-0.990,P=0.029)和CYR61蛋白(OR:0.919,95%CI:0.848-0.997,P=0.042)与强直性脊柱炎风险呈负相关,这一结果由逆方差加权方法得出,见表3。加权中位数、加权模式和MR-Egger法得出了与之相一致的结果。MR-Egger回归(P截距> 0.05)表明多效性偏向的证据较少,Cochran’s Q统计量也强有力地支持了异质性不存在的结论(P > 0.05)。此外,逐一排除分析、散点图和森林图进一步支持了孟德尔随机化估计结果的稳健性,见图2。 2.2 14个IGF家族成员对类风湿性关节炎的影响 在对工具变量进行严格的质量控制后,最终在孟德尔随机化研究中对14个IGF家族成员进行了分析。每个IGF家族成员的工具变量数量范围为2-29个。所有单核苷酸多态性的F统计量范围为19.6-450.8,均超过了阈值10,表明弱工具变量偏倚不太可能存在。 14个IGF家族成员与类风湿性关节炎风险之间关联的结果见图3。通过逆方差加权方法确定了1个IGF家族成员与类风湿性关节炎风险之间的潜在因果关联,CYR61蛋白与类风湿性关节炎风险呈负相关(OR:0.946,95%CI:0.908-0.987,P=0.011)。加权中位数法、MR-Egger回归、加权模式与简单模式法得出了与之相一致的结果。MR-Egger回归表明定向多效性的证据较少(P截距 > 0.05),Cochran’s Q统计量表明单核苷酸多态性之间不存在显著的异质性(P > 0.05)。敏感性分析(包括逐一排除分析、散点图和森林图)进一步支持了孟德尔随机化估计结果的稳健性(图2)。 2.3 14个IGF家族成员对银屑病关节炎的影响 在对工具变量进行严格的质量控制后,最终在孟德尔随机化研究中共分析了14个IGF家族成员。每个IGF家族成员的工具变量数量范围为2-29个。使用的所有单核苷酸多态性的F统计量范围为19.6-450.8,均超过了阈值10,表明弱工具变量偏倚的可能性较低。 14个IGF家族成员与银屑病关节炎风险之间关联的结果见图4。通过逆方差加权方法确定了1个IGF家族成员与银屑病关节炎风险之间的潜在因果关系,IGFBP-7通过逆方差加权方法与银屑病关节炎风险呈正相关(OR:1.104,95%CI:1.002-1.218,P=0.046)。加权中位数法、MR-Egger回归、加权模式与简单模式法得出了相一致的结果。根据MR-Egger回归(P截距 > 0.05)定向多效性的证据较少。Cochran’s Q统计量表明异质性可以忽略不计(P > 0.05)。敏感性分析(包括逐一排除分析、散点图和森林图)进一步证实了孟德尔随机化估计结果的稳健性(图2)。"

| [1] SCHER JU, UBEDA C, ARTACHO A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67(1):128-139. [2] ZHANG X, ZHANG D, JIA H, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895-905. [3] GRACEY E, BURSSENS A, CAMBRÉ I, et al. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat Rev Rheumatol. 2020;16(4):193-207. [4] RADNER H, RAMIRO S, BUCHBINDER R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondylarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1(1):CD008951. [5] KEROLA AM, KAZEMI A, ROLLEFSTAD S, et al. All-cause and cause-specific mortality in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis: a nationwide registry study. Rheumatology (Oxford). 2022;61(12):4656-4666. [6] LEROITH D, HOLLY JMP, FORBES BE. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol Metab. 2021; 52:101245. [7] KIM HS, NAGALLA SR, OH Y, et al. Identification of a family of low-affinity insulin-like growth factor binding proteins (IGFBPs): characterization of connective tissue growth factor as a member of the IGFBP superfamily. Proc Natl Acad Sci U S A. 1997;94(24):12981-12986. [8] CLEMMONS DR. Role of IGF-binding proteins in regulating IGF responses to changes in metabolism. J Mol Endocrinol. 2018;61(1):T139-T169. [9] MAZZIOTTI G, LANIA AG, CANALIS E. Skeletal disorders associated with the growth hormone-insulin-like growth factor 1 axis. Nat Rev Endocrinol. 2022;18(6):353-365. [10] ABDI E, NAJAFIPOUR H, JOUKAR S, et al. Expression of IGF-1, IL-27 and IL-35 Receptors in Adjuvant Induced Rheumatoid Arthritis Model. Iran J Immunol. 2018;15(1): 14-27. [11] RUAN X, JIN X, SUN F, et al. IGF signaling pathway in bone and cartilage development, homeostasis, and disease. FASEB J. 2024; 38(17):e70031. [12] 李冬,董晓俊,徐成栋.IGF-1介导MAPKs通路在C3H10T1/2细胞成骨分化中的作用[J].中国骨质疏松杂志,2024,30(5):668-672. [13] ERLANDSSON MC, TÖYRÄ SILFVERSWÄRD S, NADALI M, et al. IGF-1R signalling contributes to IL-6 production and T cell dependent inflammation in rheumatoid arthritis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(9):2158-2170. [14] ZUCCOLO L, HOLMES MV. Commentary: Mendelian randomization-inspired causal inference in the absence of genetic data. Int J Epidemiol. 2017;46(3):962-965. [15] HEMANI G, ZHENG J, ELSWORTH B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. [16] RICHMOND RC, DAVEY SMITH G. Mendelian Randomization: Concepts and Scope. Cold Spring Harb Perspect Med. 2022; 12(1):a040501. [17] BURGESS S, BUTTERWORTH A, THOMPSON SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658-665. [18] SKRIVANKOVA VW, RICHMOND RC, WOOLF BAR, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233. [19] SUHRE K, ARNOLD M, BHAGWAT AM, et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat Commun. 2017;8:14357. [20] SUN BB, MARANVILLE JC, PETERS JE, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558(7708):73-79. [21] DEMONTIS D, WALTERS RK, MARTIN J, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63-75. [22] GROVE J, RIPKE S, ALS TD, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51(3):431-444. [23] YU D, SUL JH, TSETSOS F, et al. Interrogating the Genetic Determinants of Tourette’s Syndrome and Other Tic Disorders Through Genome-Wide Association Studies. Am J Psychiatry. 2019;176(3):217-227. [24] DIDELEZ V, SHEEHAN N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007;16(4):309-330. [25] SYVÄNEN AC. Toward genome-wide SNP genotyping. Nat Genet. 2005;37 Suppl:S5-10. [26] BURGESS S, THOMPSON SG, CRP CHD GENETICS COLLABORATION. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755-764. [27] BOWDEN J, DEL GRECO MF, MINELLI C, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. 2019;48(3):728-742. [28] SLOB EAW, BURGESS S. A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol. 2020;44(4):313-329. [29] BOWDEN J, DAVEY SMITH G, HAYCOCK PC, et al. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016; 40(4):304-314. [30] BOWDEN J, DAVEY SMITH G, BURGESS S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. [31] BURGESS S, FOLEY CN, ALLARA E, et al. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun. 2020;11(1):376. [32] HARTWIG FP, DAVEY SMITH G, BOWDEN J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985-1998. [33] BURGESS S, THOMPSON SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377-389. [34] GRECO M FD, MINELLI C, SHEEHAN NA, et al. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015; 34(21):2926-2940. [35] MOONESI M, ZAKA KHOSRAVI S, MOLAEI RAMSHE S, et al. IGF family effects on development, stability, and treatment of hematological malignancies. J Cell Physiol. 2021;236(6):4097-4105. [36] KASPRZAK A, KWASNIEWSKI W, ADAMEK A, et al. Insulin-like growth factor (IGF) axis in cancerogenesis. Mutat Res Rev Mutat Res. 2017;772:78-104. [37] STUARD WL, TITONE R, ROBERTSON DM. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front Endocrinol (Lausanne). 2020;11:24. [38] 陈城. IL-1β、IL-4、IGF-1、IL-10在人膝骨关节炎关节软骨中的表达及意义[D].呼和浩特:内蒙古医科大学,2024. [39] WEN C, XU L, XU X, et al. Insulin-like growth factor-1 in articular cartilage repair for osteoarthritis treatment. Arthritis Res Ther. 2021;23(1):277. [40] WON JH, CHOI JS, JUN JI. CCN1 interacts with integrins to regulate intestinal stem cell proliferation and differentiation. Nat Commun. 2022;13(1):3117. [41] ZHU Y, ALMUNTASHIRI S, HAN Y, et al. The Roles of CCN1/CYR61 in Pulmonary Diseases. Int J Mol Sci. 2020;21(21):7810. [42] LAU LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci. 2011;68(19):3149-3163. [43] MACDONALD IJ, HUANG CC, LIU SC, et al. Targeting CCN Proteins in Rheumatoid Arthritis and Osteoarthritis. Int J Mol Sci. 2021;22(9):4340. [44] MIDDLETON J, ARNOTT N, WALSH S, et al. Osteoblasts and osteoclasts in adult human osteophyte tissue express the mRNAs for insulin-like growth factors I and II and the type 1 IGF receptor. Bone. 1995;16(3):287-293. [45] FAN Y, YANG X, ZHAO J, et al. Cysteine-rich 61 (Cyr61): a biomarker reflecting disease activity in rheumatoid arthritis. Arthritis Res Ther. 2019;21(1):123. [46] CHOI C, JEONG W, GHANG B, et al. Cyr61 synthesis is induced by interleukin-6 and promotes migration and invasion of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2020;22(1):275. [47] BRIGSTOCK DR, GOLDSCHMEDING R, KATSUBE KI, et al. Proposal for a unified CCN nomenclature. Mol Pathol. 2003;56(2): 127-128. [48] LIU F, YU T, LIU J, et al. IGFBP-7 secreted by adipose-derived stem cells inhibits keloid formation via the BRAF/MEK/ERK signaling pathway. J Dermatol Sci. 2023;111(1):10-19. |
| [1] | Li Jiagen, Chen Yueping, Huang Keqi, Chen Shangtong, Huang Chuanhong. The construction and validation of a prediction model based on multiple machine learning algorithms and the immunomodulatory analysis of rheumatoid arthritis from the perspective of mitophagy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-15. |
| [2] | Chen Jiayong, Tang Meiling, Lu Jianqi, Pang Yan, Yang Shangbing, Mao Meiling, Luo Wenkuan, Lu Wei, Zhou Jiatan. Based on Mendelian randomization, the causal relationship between 1400 metabolites and sarcopenia and the correlation analysis of cardiovascular disease were investigated [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-11. |
| [3] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Exploring the causal relationship between rheumatoid arthritis and coronary atherosclerosis: a Mendel randomized study involving serum metabolites and inflammatory factors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [4] | Han Haihui, Ran Lei, Meng Xiaohui, Xin Pengfei, Xiang Zheng, Bian Yanqin, Shi Qi, Xiao Lianbo. Targeting fibroblast growth factor receptor 1 signaling to improve bone destruction in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1905-1912. |
| [5] | Dong Tingting, Chen Tianxin, Li Yan, Zhang Sheng, Zhang Lei. Causal relationship between modifiable factors and joint sports injuries [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1953-1962. |
| [6] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [7] |
Zhao Wensheng, Li Xiaolin, Peng Changhua, Deng Jia, Sheng Hao, Chen Hongwei, Zhang Chaoju, He Chuan.
Gut microbiota and osteoporotic fractures #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1296-1304.
|
| [8] | Ma Haoyu, Qiao Hongchao, Hao Qianqian, Shi Dongbo. Causal effects of different exercise intensities on the risk of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1305-1311. |
| [9] | Li Jiatong, Jin Yue, Liu Runjia, Song Bowen, Zhu Xiaoqian, Li Nianhu . Association between thyroid function levels and phenotypes associated with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1312-1320. |
| [10] | Han Haihui, Meng Xiaohu, Xu Bo, Ran Le, Shi Qi, Xiao Lianbo. Effect of fibroblast growth factor receptor 1 inhibitor on bone destruction in rats with collagen-induced arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 968-977. |
| [11] | Wang Yuru, Li Siyuan, Xu Ye, Zhang Yumeng, Liu Yang, Hao Huiqin. Effects of wogonin on joint inflammation in collagen-induced arthritis rats via the endoplasmic reticulum stress pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1026-1035. |
| [12] | Wu Guangtao, Qin Gang, He Kaiyi, Fan Yidong, Li Weicai, Zhu Baogang, Cao Ying . Causal relationship between immune cells and knee osteoarthritis: a two-sample bi-directional Mendelian randomization analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1081-1090. |
| [13] | Wang Tao, Wang Shunpu, Min Youjiang, Wang Min, Li Le, Zhang Chen, Xiao Weiping. Causal relationship between gut microbiota and rheumatoid arthritis: data analysis in European populations based on GWAS data [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7663-7668. |
| [14] | Han Jie, Pan Chengzhen, Shang Yuzhi, Zhang Chi. Identification of immunodiagnostic biomarkers and drug screening for steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7690-7700. |
| [15] | Zhu Jiaping, Gao Bo, Lou Chunbiao, Yang Fengyong, Yang Kun. Monomeric traditional Chinese medicine in the treatment of rheumatoid arthritis: regulation of T cell balance [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6955-6962. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||