Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (29): 6187-6197.doi: 10.12307/2025.772
Previous Articles Next Articles
Therapeutic effect of Cornus Cervi Colla on steroid-induced osteonecrosis of the femoral head in rat models: fecal metabolomics analysis
Chai Jinlian1, Sun Tiefeng2, Li Wei3, Zhang Bochun3, Li Guangzheng4, Zhou Zhongqi4, Liang Xuezhen3, 5, Wang Ping2
- 1College of Pharmacy, 4College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China; 2Shandong Provincial Research Institute of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China; 3First College of Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan 250355, Shandong Province, China; 5Department of Orthopedic Microsurgery, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China
-
Received:2024-09-18Accepted:2024-11-12Online:2025-10-18Published:2025-03-06 -
Contact:Liang Xuezhen, MD, Associate professor, Master’s supervisor, First College of Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan 250355, Shandong Province, China; Department of Orthopedic Microsurgery, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China Corresponding author: Wang Ping, PhD, Researcher, Master’s supervisor, Shandong Provincial Research Institute of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China -
About author:Chai Jinlian, MS, College of Pharmacy, Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China -
Supported by:National Natural Science Foundation of China, No. 82205154 (to LXZ); Shandong Natural Science Foundation (Youth Project), No. ZR2021QH004, ZR2024MH156 (to LXZ); Shandong Natural Science Foundation, No. ZR2020MH386 (to WP)
CLC Number:
Cite this article
Chai Jinlian, Sun Tiefeng, Li Wei, Zhang Bochun, Li Guangzheng, Zhou Zhongqi, Liang Xuezhen, Wang Ping. Therapeutic effect of Cornus Cervi Colla on steroid-induced osteonecrosis of the femoral head in rat models: fecal metabolomics analysis[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6187-6197.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
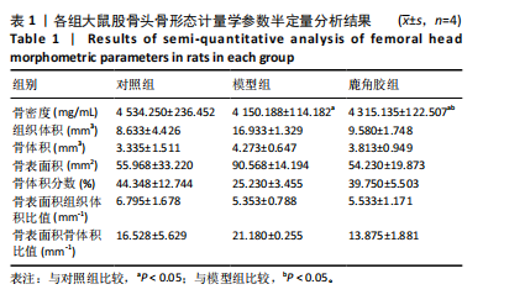
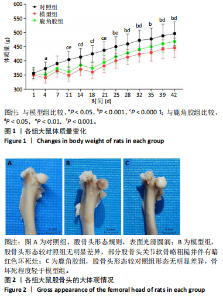
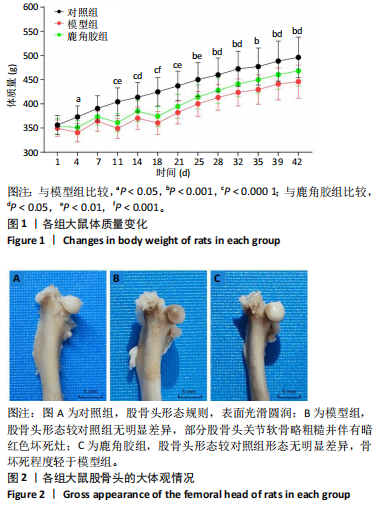
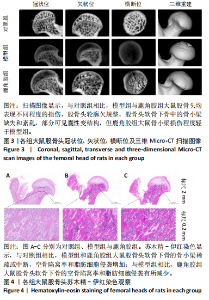
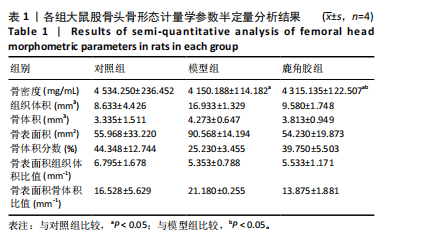
2.1 实验动物数量分析 30只SD大鼠全部进入结果分析。 2.2 各组大鼠基本状态 对照组大鼠毛色和光泽正常,饮食、排泄和精神状态良好,体质量随时间逐渐增加。在甲泼尼龙琥珀酸钠注射期间,模型组部分大鼠精神状态不佳,食欲下降,毛色灰暗稀释,大便稀软,尿量多,体质量出现下降,停止注射后体质量上升,第3周注射完成后食欲尚可,毛色、光泽恢复正常,体质量呈现持续增长状态,但明显低于对照组。在甲泼尼龙琥珀酸钠注射期间,鹿角胶组大鼠食欲尚可,毛色略灰暗稀疏,大便轻度稀软,尿量稍多,体质量也出现一定程度的下降,停止注射后体质量上升,第3周注射完成后食欲恢复正常,毛色、光泽逐渐恢复正常,体质量呈现持续增长状态,整体增长态势介于对照组与模型组之间。各组大鼠体质量变化见图1。 2.3 各组大鼠股骨头大体形态观察结果 对照组股骨头形态规则,表面光滑圆润,呈现健康的白色或略带黄色;模型组股骨头形态较对照组无明显差异,部分股骨头关节软骨略粗糙,呈现暗且薄的软骨表面,并伴有暗红色坏死灶;鹿角胶组股骨头形态较对照组形态无明显差异,软骨表面偶见不均匀的灰白色,但骨坏死程度无模型组严重,见图2。 2.4 各组大鼠股骨头Micro-CT扫描及骨形态计量学参数分析结果 Micro-CT扫描图像显示,与对照组相比,模型组与鹿角胶组大鼠股骨头均表现不同程度的损伤,股骨头轮廓欠规整,股骨头软骨下骨中的骨小梁缺失和紊乱,部分可见囊性变结构,但鹿角胶组大鼠骨小梁损伤程度轻于模型组,见图3。 骨形态计量学参数中骨密度半定量分析结果显示,模型组和鹿角胶组大鼠骨密度均明低于对照组(P < 0.05),鹿角胶组大鼠骨密度高于模型组(P < 0.05),各组骨形态计量学参数中的其他参数(包括组织体积、骨体积、骨表面积、骨体积分数、骨表面积组织体积比值、骨表面积骨体积比值)比较差异均无显著性意义(P > 0.05),见表1。"
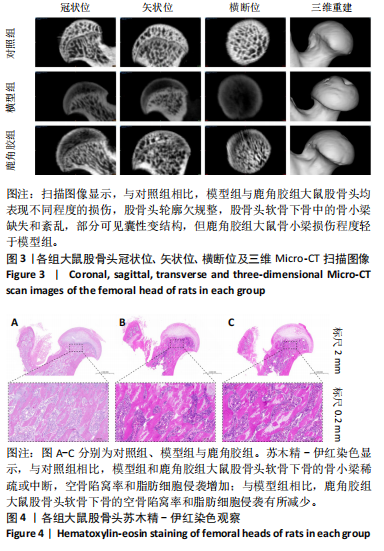
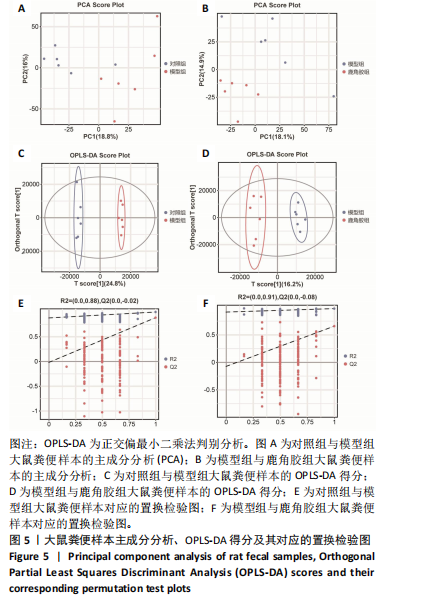
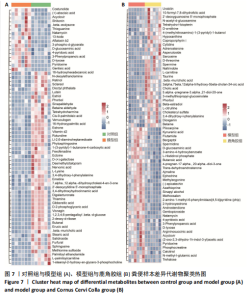
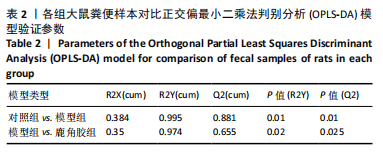
2.5 各组大鼠股骨头苏木精-伊红染色结果 苏木精-伊红染色显示,与对照组相比,模型组和鹿角胶组大鼠股骨头软骨下骨的骨小梁稀疏或中断,空骨陷窝率和脂肪细胞侵袭增加;与模型组相比,鹿角胶组大鼠股骨头软骨下骨的空骨陷窝率和脂肪细胞侵袭有所减少,见图4。 2.6 各组大鼠粪便代谢物分析 2.6.1 多元统计分析 采用主成分分析(principal component analysis,PCA)和正交偏最小二乘法判别分析(orthogonal partial least squares discriminant analysis,OPLS-DA)对结果进行分析。PCA分析显示各组样本表现组内聚类及组间离散,各组样本之间具有差异;进一步对比各组OPLS-DA模型的相关评价参数表明,代谢物表达量与样品类别之间建立的关系模型可靠;OPLS-DA评分图显示了对照组、模型组、鹿角胶组之间的样本对比区分非常显著。OPLS-DA置换检验表明各组OPLS-DA模型不存在过拟合,具备较好的有效性及稳健性。各组对比的OPLS-DA模型相关验证参数见表2。PCA分析、OPLS-DA评分和置换检验见图5。"

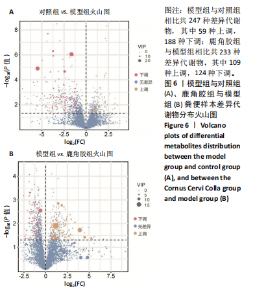
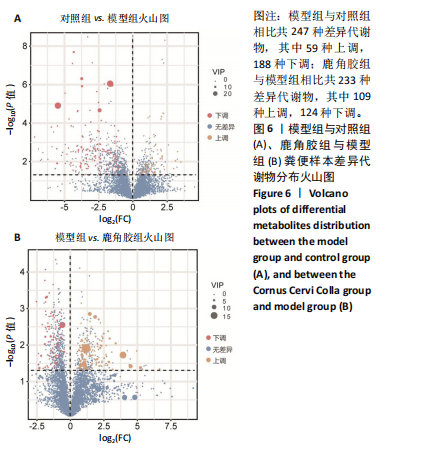
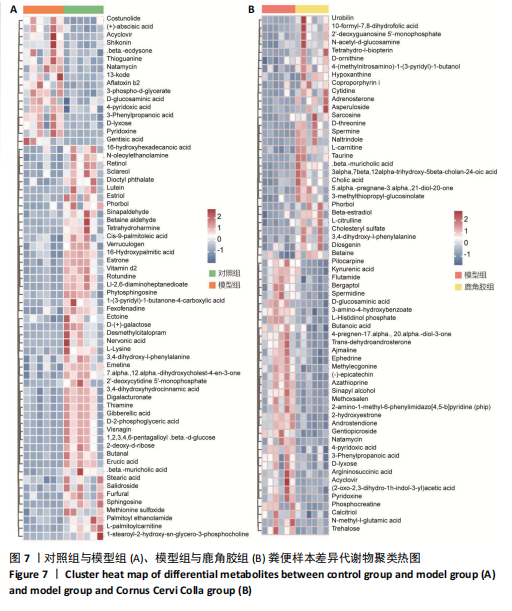
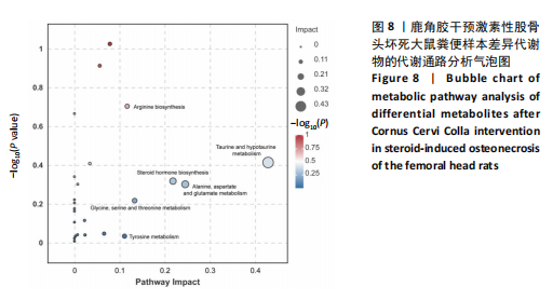
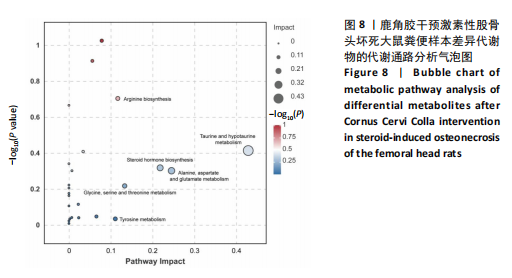
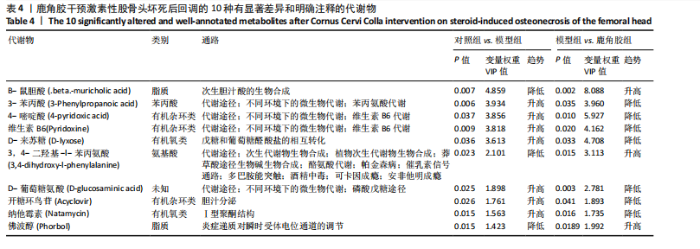
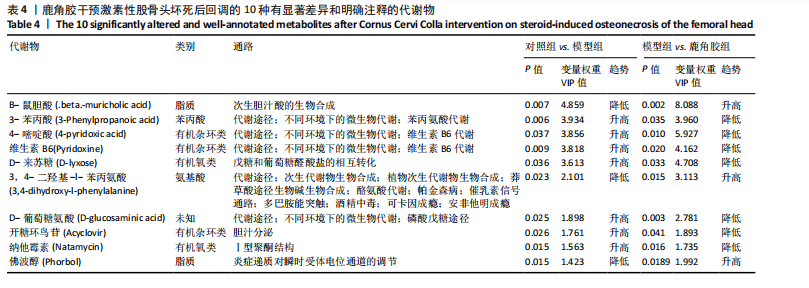
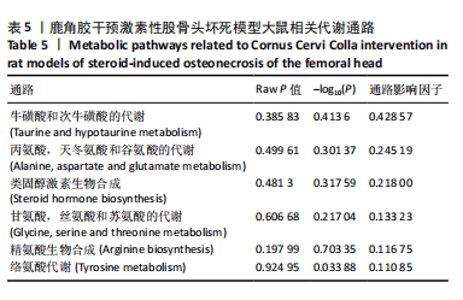
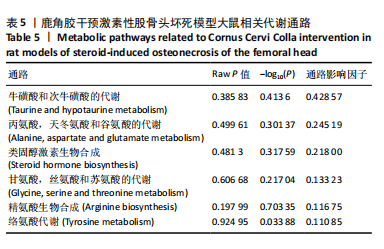
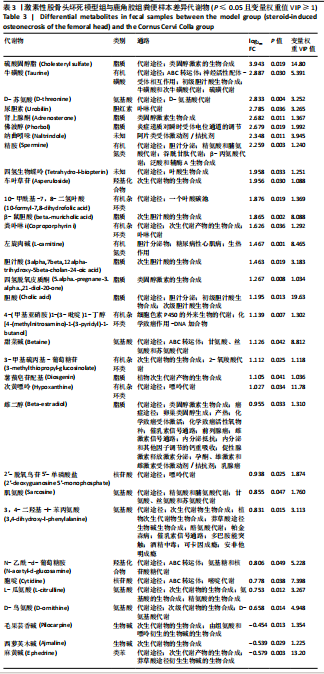
2.6.2 差异代谢物分析 对各组之间的差异代谢物丰度进行差异倍数计算,并根据变量权重值(VIP值)≥1、P≤0.05,利用R包ggplot2绘制差异代谢物的火山图,可以看出在组间差异代谢物分布情况,见图6。 此次研究设定OPLS-DA模型中的VIP≥1、P≤0.05。经筛选,模型组与对照组相比共247种差异代谢物,其中59种上调,188种下调。鹿角胶组与模型组相比共233种差异代谢物,其中109种上调,124种下调。根据精准分子质量及数据库比对,最终确定对照组与模型组中有65个具有显著差异和明确注释的代谢物;模型组与鹿角胶组中有64个。对上述差异代谢物进行聚类分析得到图7。聚类热图显示各组组内数据重复性较好。同模型组相比,鹿角胶组粪便样本中有30个代谢物上调,34个代谢物下调,各代谢物详细信息见表3。 由表3可以看出,脂质和氨基酸是模型组与鹿角胶组最主要的几类差异代谢产物。脂质代谢中与胆汁酸分泌、初级胆汁酸生物合成、次级胆汁酸生物合成途径相关的代谢物B-鼠胆酸(log2_FC=1.865)、胆汁酸(log2_FC=1.463)以及胆酸(log2_FC=1.195),在鹿角胶组粪便样本中的含量显著高于模型组;与炎症递质对瞬时受体电位通道调节途径相关的佛波醇(log2_FC=2.679),在鹿角胶组粪便样本中的含量显著高于模型组;与丁酸代谢途径相关的丁酸(log2_FC=-0.699),与甲状旁腺激素合成、分泌和作用途径相关的骨化三醇(log2_FC=-0.719),在鹿角胶组粪便样本中的含量均显著低于模型组。 氨基酸代谢中与D-氨基酸代谢途径相关的代谢物D-苏氨酸(log2_FC=2.822)和D-鸟氨酸(log2_FC=0.658),在鹿角胶组在鹿角胶组粪便样本中的含量显著高于模型组,以及与甘氨酸、丝氨酸和苏氨酸代谢途径相关的甜菜碱(log2_FC=1.126)、肌氨酸(log2_FC=0.855),在鹿角胶组粪便样本中的含量显著高于模型组;与丙氨酸、天冬氨酸和谷氨酸的代谢途径相关的代谢物精氨酸琥珀酸(log2_FC=-1.059),在鹿角胶组粪便样本中的含量显著低于模型组。 值得注意的是,与初级胆汁酸生物合成、牛磺酸和次牛磺酸代谢途径相关的牛磺酸(log2_FC=2.887),在鹿角胶组粪便样本中的含量要显著高于模型组。 对64个代谢物的VIP值进行降序排列,可以看出胆酸、硫酸固醇脂、麻黄碱、次黄嘌呤、甜菜碱、左旋肉碱、B-鼠胆酸、胞啶、4-嘧啶酸、牛磺酸、N-乙酰-d-葡萄糖胺、丁酸是影响度最高的几种代谢物(VIP > 5),其中胆酸、左旋肉碱、B-鼠胆酸、牛磺酸均与胆汁代谢途径相关。 另外,在对照组vs. 模型组65个有显著差异和明确注释的代谢物中,有10种在鹿角胶应用后有明显的回调,见表4。 2.6.3 差异代谢物通路分析 采用KEGG数据库(http://metpa.metabolomics.ca)与MetPA数据库(http://metpa.metabolomics.ca)对鹿角胶组干预激素性股骨头坏死模型大鼠后的233种差异代谢物进行映射、富集分类,共得到73条显著富集的代谢通路。根据富集和影响因子分析得到代谢通路影响因子气泡图,见图8。其中,6条具有显著性功能的代谢通路(通路影响因子 > 0.1),分别为牛磺酸和次牛磺酸的代谢、丙氨酸,天冬氨酸和谷氨酸的代谢、类固醇激素生物合成、甘氨酸,丝氨酸和苏氨酸的代谢、精氨酸生物合成、络氨酸代谢,见表5。在代谢调控网络中的重要性最高的是牛磺酸和次牛磺酸的代谢途径(通路影响因子=0.428 57),从该通路的显著性通路图中可以看出,鹿角胶组牛磺酸的上调是影响该代谢途径的核心环节,而与其密切相关的初级胆汁酸生物合成途径当中,初级胆汁酸的上调是引起牛磺酸上调的关键因素。"
| [1] CHEN K, LIU Y, HE J, et al. Steroid-induced osteonecrosis of the femoral head reveals enhanced reactive oxygen species and hyperactive osteoclasts. Int J Biol Sci. 2020;16(11):1888-1900. [2] HUANG C, WEN Z, NIU J, et al. Steroid-Induced Osteonecrosis of the Femoral Head: Novel Insight Into the Roles of Bone Endothelial Cells in Pathogenesis and Treatment. Front Cell Dev Biol. 2021;9:777697. [3] WANG Y, LI D, CHEN H, et al. Accumulation of Fat Not Responsible for Femoral Head Necrosis, Revealed by Single-Cell RNA Sequencing: A Preliminary Study. Biomolecules. 2023;13(1):171. [4] CHANG C, GREENSPAN A, GERSHWIN ME. The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J Autoimmun. 2020;110:102460. [5] ZHANG X, YANG Z, XU Q, et al. Dexamethasone Induced Osteocyte Apoptosis in Steroid-Induced Femoral Head Osteonecrosis through ROS-Mediated Oxidative Stress. Orthop Surg. 2024;16(3):733-744. [6] WANG A, REN M, WANG J. The pathogenesis of steroid-induced osteonecrosis of the femoral head: A systematic review of the literature. Gene. 2018;671:103-109. [7] FANG S, HE T, YOU M, et al. Glucocorticoids promote steroid-induced osteonecrosis of the femoral head by down-regulating serum alpha-2-macroglobulin to induce oxidative stress and facilitate SIRT2-mediated BMP2 deacetylation. Free Radic Biol Med. 2024;213:208-221. [8] 孙铁锋,王平.鹿角胶-壳聚糖细胞支架修复大鼠股骨头缺血性坏死[J].中成药,2015,37(11):2337-2341. [9] TAO SC, YUAN T, RUI BY, et al. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7(3):733-750. [10] 武瑞骐,崔伟,杨启培,等.激素性股骨头坏死的中医药治疗机制[J].中国组织工程研究,2023,27(17):2763-2771. [11] 郭雪峰,任艳玲,于睿,等.基于“久病入络”理论探讨“从虚而始,因虚致瘀,瘀痹骨络”的激素性股骨头坏死核心病机观[J].中华中医药学刊,2024,42(6):191-194. [12] 黄为,刘琴,韦宗波,等.激素性股骨头坏死研究现状及趋势可视化分析[J].世界中西医结合杂志,2023,18(2):225-235+243. [13] 张绵钰,韩杰,曾浩,等.中药调控成骨细胞铁死亡治疗激素性股骨头坏死[J].中国组织工程研究,2025,29(1):185-192. [14] 孙昭杰,张宁,李明阳,等.激素性股骨头坏死的中西医治疗进展[J].中医药学报,2023,51(6):105-109. [15] 郭雪峰,于睿,任艳玲,等.基于“成骨-成血管耦联”理论探讨中医药防治激素性股骨头坏死的科学内涵[J].中华中医药学刊, 2024,42(7):56-60. [16] 杨小瑞,曹林忠,胡康一,等.细胞焦亡在骨代谢异常疾病中的研究[J].中国骨质疏松杂志,2024,30(1):124-128. [17] 汪亮,刘耀升,刘蜀彬.糖皮质激素诱导股骨头坏死发病机制的研究进展[J].中华损伤与修复杂志(电子版),2015,10(5):439-445. [18] SUN K, XUE Y, ZHANG X, et al. Tanshinone I alleviates steroid-induced osteonecrosis of femoral heads and promotes angiogenesis: in vivo and in vitro studies. J Orthop Surg Res. 2023;18(1):474. [19] ZHAO P, ZHAO S, ZHANG J, et al. Molecular Imaging of Steroid-Induced Osteonecrosis of the Femoral Head through iRGD-Targeted Microbubbles. Pharmaceutics. 2022;14(9):1898. [20] NAN K, PEI JP, FAN LH, et al. Resveratrol prevents steroid-induced osteonecrosis of the femoral head via miR-146a modulation. Ann N Y Acad Sci. 2021;1503(1):23-37. [21] WANG F, MIN H S, SHAN H, et al. IL-34 Aggravates Steroid-Induced Osteonecrosis of the Femoral Head via Promoting Osteoclast Differentiation. Immune Netw. 2022;22(3):e25. [22] PHAN V, BLYDT-HANSEN T, FEBER J, et al. Skeletal findings in the first 12 months following initiation of glucocorticoid therapy for pediatric nephrotic syndrome. Osteoporos Int. 2014;25(2):627-637. [23] 胡亚楠,杜海涛,于洋,等.鹿角胶体内分布及入血入骨成分的示踪动力学分析[J].中国组织工程研究,2024,28(28):4441-4446. [24] 胡亚楠,王平,杜海涛,等.血清蛋白质组学技术发现鹿角胶治疗维甲酸致股骨头坏死模型大鼠的机制[J].中国组织工程研究, 2022,26(14):2243-2251. [25] 王平,张会敏,李刚.鹿角多肽对骨髓间充质干细胞的影响[J].中华中医药杂志,2018,33(12):5644-5647. [26] 李纪书,郭成龙,任行全,等.激素性股骨头坏死相关信号通路及发病机制研究概述[J].现代中医药,2024,44(1):5-10. [27] 梁学振,杨曦,李嘉程,等.补肾活血胶囊对激素性股骨头坏死大鼠骨修复和脂肪形成的影响[J].中华中医药杂志,2022,37(3): 1763-1768. [28] 汤朔,侯德才.股骨头坏死动物模型构建:如何更接近临床应用[J].中国组织工程研究,2021,25(29):4691-4696. [29] 石包圣,闫国珍,李爱华,等.早期激素性股骨头坏死动物模型制备的实验研究[J].包头医学院学报, 2021,37(5):109-112. [30] 李瑞琦,张国平,李宜炯,等.激素性股骨头坏死模型:不同构建技术分析[J].中国组织工程研究,2013,17(37):6676-6681. [31] 汪轩,张琳,邢婧,等.激素剂量对激素性股骨头坏死模型建立的影响及系统评价[J].浙江中医药大学学报,2016,40(1):19-25. [32] 佟鹏,王洋,梁瀛.激素性股骨头缺血性坏死动物模型的建立及综合评估[J].中国组织工程研究,2018,22(32):5169-5174. [33] 周磊,翁习生.激素性股骨头坏死动物模型的选择与应用[J].中国矫形外科杂志,2013,21(21):2155-2158. [34] 王岩,马剑雄,董本超,等.激素性股骨头坏死动物模型的研究进展[J].生物医学工程与临床,2021,25(5):650-656. [35] HADJIGEORGIOU G, DARDIOTIS E, DARDIOTI M, et al. Genetic association studies in osteonecrosis of the femoral head: mini review of the literature. Skeletal Radiol. 2008;37(1):1-7. [36] ZHANG YW, SONG PR, WANG SC, et al. Diets intervene osteoporosis via gut-bone axis. Gut Microbes. 2024;16(1):2295432. [37] 王博,景少博,徐世红,等.微生物、肠脑轴在骨质疏松症中的作用及中药干预研究进展[J].实用中医内科杂志,2024,38(4):40-43+150. [38] KAZEMIAN N, MAHMOUDI M, HALPERIN F, et al. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome. 2020;8(1):36. [39] HEINKEN A, RAVCHEEV DA, BALDINI F, et al. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome. 2019;7(1):75. [40] ASANO K, SUZUKI T, SAITO A, et al. Metabolic and chemical regulation of tRNA modification associated with taurine deficiency and human disease. Nucleic Acids Res. 2018;46(4):1565-1583. [41] PéREZ-HERNÁNDEZ E, PASTRANA-CARBALLO JJ, GóMEZ-CHáVEZ F, et al. A Key Metabolic Regulator of Bone and Cartilage Health. Endocrinol Metab (Seoul). 2022;37(4):559-574. [42] HIRATA H, UEDA S, ICHISEKI T, et al. Taurine Inhibits Glucocorticoid-Induced Bone Mitochondrial Injury, Preventing Osteonecrosis in Rabbits and Cultured Osteocytes. Int J Mol Sci. 2020;21(18):6892. |
| [1] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [2] | Yu Qinghe, Cai Ziming, Wu Jintao, Ma Pengfei, Zhang Xin, Zhou Longqian, Wang Yakun, Lin Xiaoqin, Lin Wenping. Vanillic acid inhibits inflammatory response and extracellular matrix degradation of endplate chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6391-9397. |
| [3] | Fan Jiaxin, Jia Xiang, Xu Tianjie, Liu Kainan, Guo Xiaoling, Zhang Hui, Wang Qian . Metformin inhibits ferroptosis and improves cartilage damage in osteoarthritis model rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6398-6408. |
| [4] | Zhou Ying, Tian Yong, Zhong Zhimei, Gu Yongxiang, Fang Hao. Inhibition of tumor necrosis factor receptor associated factor 6 regulates mTORC1/ULK1 signaling and promotes autophagy to improve myocardial injury in sepsis mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6434-6440. |
| [5] | Wang Wanchun, , Yi Jun, Yan Zhangren, Yang Yue, Dong Degang, Li Yumei. 717 Jiedu Decoction remodels homeostasis of extracellular matrix and promotes repair of local injured tissues in rats after Agkistrodon halys bite [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6457-6465. |
| [6] | Zhang Xin, Guo Baojuan, Xu Huixin, Shen Yuzhen, Yang Xiaofan, Yang Xufang, Chen Pei. Protective effects and mechanisms of 3-N-butylphthalide in Parkinson’s disease cell models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6466-6473. |
| [7] | Zhang Songjiang, Li Longyang, Zhou Chunguang, Gao Jianfeng. Central anti-inflammatory effect and mechanism of tea polyphenols in exercise fatigue model mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6474-6481. |
| [8] | Hu Shujuan, Liu Dang, Ding Yiting, Liu Xuan, Xia Ruohan, Wang Xianwang. Ameliorative effect of walnut oil and peanut oil on atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6482-6488. |
| [9] | Zhang Jian, Cai Feng, Li Tingwen, Ren Pengbo. Fatigue gait recognition of athletes based on fish swarm algorithm [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6489-6498. |
| [10] | Zhang Zihan¹, Wang Jiaxin¹, Yang Wenyi², Zhu Lei¹. Regulatory mechanism of exercise promoting mitochondrial biogenesis in skeletal muscle [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6499-6508. |
| [11] | Wang Jianxu, Dong Zihao, Huang Zishuai, Li Siying, Yang Guang. Interaction between immune microenvironment and bone aging and treatment strategies [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6509-6519. |
| [12] | Zhang Bochun, Li Wei, Li Guangzheng, Ding Haoqin, Li Gang, Liang Xuezhen, . Association between neuroimaging changes and osteonecrosis: a large sample analysis from UK Biobank and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6574-6582. |
| [13] | Xu Zhi, Chen Yundong, Sun Yujie, Gong Xiaonan, Li Yuwan. Data of spinal osteosarcoma patients in United States based on SEER database: construction and validation of a prediction model for treatment outcomes and prognosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6583-6590. |
| [14] | Zhao Xiaoxuan, Liu Shuaiyi, Xing Zheng, Li Qingwen, Chu Xiaolei, Li Qi. Research hotspots and trends in application of tissue engineering in peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6591-6600. |
| [15] | Wu Xiaochou, Wang Huiying, Wang Jie, Zhang Caifeng, Hou Yanyun, Jin Bo. Protective mechanism of tanshinone IIA in mouse ovarian cryopreservation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6198-6204. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||