Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (24): 3910-3914.doi: 10.12307/2024.615
Previous Articles Next Articles
Role and mechanism of interleukin-8 in bone regeneration
Luo Peng1, Wang Yi1, Wang Ansu1, Dang Yi1, Ma Yaping1, Zhang Yi2, Wang Xin1
- 1Department of Orthopaedic Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China; 2School of Public Health, Zunyi Medical University, Zunyi 563000, Guizhou Province, China
-
Received:2023-07-07Accepted:2023-08-18Online:2024-08-28Published:2023-11-22 -
Contact:Wang Xin, MD, Chief physician, Doctoral supervisor, Department of Orthopaedic Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
About author:Luo Peng, Master candidate, Department of Orthopaedic Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China Wang Yi, Master candidate, Department of Orthopaedic Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 31960209; Outstanding Youth Scientific Fund of Guizhou Province, No. [2023]039; a grant from Guizhou Science and Technology Department, No. [2020]1Y093; Future Eminent Clinician Plan of Zunyi Medical University, No. 2022-02; Doctoral Science Research Startup Funding of Zunyi Medical University, No. 2017-01 (all to WX); National Natural Science Foundation of China, No. 82060620; a grant from Guizhou Science and Technology Department, No. ZK[2023]502 (all to ZY); Zunyi Science and Technology Fund Project, No. HZ[2021]40 (to MYP)
CLC Number:
Cite this article
Luo Peng, Wang Yi, Wang Ansu, Dang Yi, Ma Yaping, Zhang Yi, Wang Xin. Role and mechanism of interleukin-8 in bone regeneration[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(24): 3910-3914.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
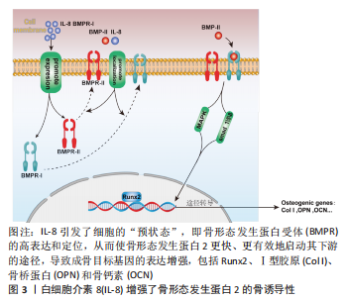
2.1 IL-8与骨代谢 骨组织是一种动态矿化组织,具有强大的再生修复的能力,其通过破骨细胞促进骨吸收和成骨细胞促进成骨来维持体内平衡[5],该平衡一旦被打破就会造骨骼结构异常和功能障碍,产生代谢性骨疾病如骨质疏松症等[6]。成骨细胞来源于人体MSCs,其对于维持骨量和骨骼发育是不可或缺的,可分化为骨细胞并调控骨基质矿化;而破骨细胞则是一种由单核巨噬细胞谱系中的造血干细胞发育而成的多核巨细胞,其主要功能是负责骨吸收[7]。 IL-8是趋化因子家族的成员,最初被定义为中性粒细胞的趋化剂[8],可由多种细胞产生,如巨噬细胞、上皮细胞、气道平滑肌细胞和内皮细胞[9]。IL-8过表达不仅与肿瘤生长、血管生成和转移相关[10],还在破骨细胞形成和骨吸收中起着关键作用[8]。有研究报道,IL-8通过自分泌和旁分泌方式调节MSCs的功能,促进MSCs对骨再生的治疗作用[11]。此外,IL-8直接增强内皮细胞的生存、增殖并调节血管生成[12]。研究表明,IL-8能够促进成骨细胞和骨母细胞的增殖和分化,增加骨形成[13-15]。 IL-8对骨的作用是多样的,除在成骨上有一定作用外,还能促进人类骨髓间充质干细胞向破骨细胞分化,并增强破骨细胞的活性[16]。同时,IL-8也能够促进破骨细胞的迁移和侵袭,增加骨吸收程度[17]。 总之,IL-8在骨代谢中发挥着重要的作用,既可以促进骨形成、又能增加骨吸收和调节骨髓间充质干细胞的增殖和分化。IL-8通过作用于成骨细胞和破骨细胞,影响骨吸收和骨形成,并参与了多种信号通路的调节。以往对于IL-8在骨代谢方面的研究不够深入,未细致阐明其中机制,且从研究结果来看,IL-8对骨代谢的作用是双向的,还需要在未来的研究中进一步探讨IL-8在骨代谢的具体作用机制和发生双向作用的原因以及临床应用前景,完善IL-8在骨代谢中的作用研究,为后续研究提供理论参考。 2.1.1 IL-8对MSCs的作用 MSCs具有自我更新和多种分化潜能,其能够分化成各种类型的细胞,包括成骨细胞、软骨细胞、脂肪细胞和成肌细胞等[18]。MSCs来源于骨髓、脂肪、血液等多种组织,是骨组织修复再生的重要组成部分[19]。无论是在骨生长阶段还是骨重塑阶段,MSCs都参与以下2种骨形成模式:一是膜内骨化,MSCs募集后直接骨化或分化为成熟的成骨细胞;二是软骨内骨化,即MSCs分化为成软骨细胞沉积软骨,其钙化后再重塑成骨[20]。因此,MSCs在良好的骨再生微环境中发挥着重要作用。 既往研究报道,IL-8促进MSCs的增殖和动员[21]。IL-8受体α(CXCR1)和IL-8受体β(CXCR2)都是G蛋白偶联受体家族成员,其具有相似的跨膜结构。MSCs可以分泌IL-8,而IL-8与其受体CXCR1或CXCR2结合并激活该受体,通过自分泌或旁分泌方式调节MSCs的功能[11]。由此可见,IL-8可以通过MSCs发挥成骨作用。有研究指出,IL-8通过作用于CXCR1或CXCR2受体激活细胞内磷酸肌醇3-激酶/蛋白激酶B(phosphoinositide 3-kinase/protein kinase B,PI3K/AKT)信号通路,从而发挥作用[22]。信号通路参与细胞增殖、分化、侵袭和凋亡等过程,PI3K/AKT信号通路主要是由于PI3K的激活导致AKT磷酸化,AKT通过抑制促凋亡蛋白的活性和上调抗凋亡蛋白的表达来促进细胞存活[23]。YANG等[11]通过实验检测IL-8/CXCR1/CXCR2/PI3K/AKT对MSCs迁移潜能的影响,发现IL-8能通过CXCR2介导的PI3K/AKT信号通路触发MSCs体外迁移,且IL-8促进小鼠骨缺损成骨,该实验结果表明IL-8通过CXCR2介导的PI3K/AKT信号通路诱导MSCs向软骨细胞分化,从而促进成骨。此外,SHEN等[24]在缺氧环境下,发现IL-8可以活化AKT通路,通过激活下游的mTOR蛋白促进MSCs自噬的发生,增强细胞自噬能力,可使MSCs获得更多能量和营养物质,进一步提高MSCs的增殖能力。综上所述,IL-8促进MSCs自噬、增殖及分化,从而促进骨再生。 2.1.2 IL-8对成骨细胞的作用 成骨细胞是来源于MSCs的一种特化性细胞,在骨缺损修复过程当中是主要负责骨产生和重塑的一种间充质衍生细胞,其通过产生不同的细胞外基质蛋白来调节骨骼结构和骨基质矿化,此外,成骨细胞还能产生不同的细胞因子甚至直接诱导骨的形成[25]。虽然成骨细胞仅占骨细胞的4%-5%[26],但是成骨细胞是构建骨骼中非常重要的细胞,在其分化的各个阶段的调节都需要严格把控,以保证适当的骨骼成长和平衡。 在骨缺损的局部由于在愈合早期缺损部位血肿的形成会将局部包裹起来形成一个密闭的空间,造成缺损部位缺血、缺氧。NIU等[27]认为缺氧可调节血管生成-成骨耦合过程,并检测到在缺氧情况下IL-8的mRNA水平和其蛋白表达水平明显增加数倍之多,通过实验证明在缺氧环境下成骨细胞确实分泌IL-8活动增强,实验中还发现了重组人IL-8在0.01 ng/mL浓度时可显著刺激成骨细胞MG-63和单核细胞RAW264.7的增殖,并且经过IL-8处理过后的细胞中,其内皮细胞的增殖能力和迁移能力也有相应的提升。而DA CRUZ等[28]的实验也证明一定的实验技术在促进成骨细胞增殖、分化的同时也促进IL-8的分泌增加。这些结果都在提示:在成骨微环境中,IL-8与成骨细胞是可以和谐共存,并且IL-8的表达增加极可能有利于成骨细胞的增殖和分化。为探究IL-8对成骨的确切影响,有人将IL-8吸附到含有壳聚糖微球的介孔生物活性玻璃支架中,制成含有骨形态发生蛋白2和IL-8的复合支架,通过该支架不断释放IL-8来探究IL-8对骨形态发生蛋白骨诱导性的影响,实验结果显示:支架持续释放IL-8将会明显增加骨形态发生蛋白2的骨诱导性,对成骨有较强的促进作用,该支架由于IL-8的加入高效加速了整个再生的过程,早期骨形成量和再生量得到明显提高;并且在持续释放的第5天显示出矿物质沉积的效果最强[29]。在骨早期愈合中干细胞募集阶段,IL-8在体外对骨髓MSCs的趋化作用更强,并且体内募集骨髓MSCs的效率高于骨形态发生蛋白2[2]。在这项研究中,虽然 IL-8 缺乏减弱整个过程的成骨能力,但可以协同骨形态发生蛋白2增强软骨内骨化。一方面,IL-8 在体内表现出比骨形态发生蛋白2更好的软骨形成能力,体外软骨形成标记物表达的上调;另一方面,IL-8还从细胞水平上增加了骨形态发生蛋白2及其受体的结合效率,从而增强了骨形态发生蛋白2的骨诱导性。因此,当一起应用时,IL-8首先通过提供丰富的软骨细胞和软骨组织赋予软骨内骨化的“准备状态”,当其与骨形态发生蛋白2接触,软骨细胞和软骨组织进一步转变为编织骨。在这种状态中,细胞暴露于骨形态发生蛋白2可以快速有效地启动骨形态发生蛋白信号及其下游通路的转导。因此,转录因子Runx2的激活得到增强和延长,从而上调骨基质成熟中的关键蛋白Ⅰ型胶原的表达,并最终导致骨矿化标志物表达的显著增加,从而助力骨再生,详见图3。"

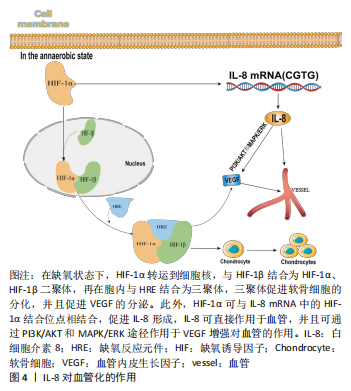
综上所述,目前多项研究显示出在早期骨再生阶段中均有IL-8的分泌,且该物质的分泌对骨再生是有一定协同作用的,并且在后期研究中也证实IL-8可为软骨内化骨做好铺垫,并且在此过程中能增强骨形态诱导蛋白的效率,而其中的具体机制却未见文献报道,此处有待进一步研究阐明。 2.1.3 IL-8对破骨细胞的作用 破骨细胞来自造血祖细胞,是造血前体细胞衍生的多核细胞,其通过巨噬细胞集落刺激因子和核因子κB配体受体致活剂(receptor activator of nuclear factor k app a-B ligand,RANKL)从单核/巨噬细胞系细胞分化而来,具有骨吸收功能。破骨细胞的分化、存活和活性主要受2种关键细胞因子调控,即巨噬细胞集落刺激因子和RANKL。巨噬细胞集落刺激因子与集落刺激因子1受体结合可激活PI3K和生长因子受体结合蛋白2,进而在前破骨细胞或成熟破骨细胞中诱导AKT和细胞外信号调节激酶(extracellular signal-regulated kinase,ERKs)信号,从而促进骨吸收[30]。RANKL是破骨细胞形成不可或缺的因子,是一种表达于成骨细胞表面的Ⅱ型跨膜蛋白,它通过刺激破骨细胞祖细胞表达的核转录因子κB受体活化因子(receptor activator of nuclear factor kappa B,RANK),与RANK受体结合,从而控制破骨细胞的分化成熟,介导骨的吸收[31]。 早期1995年FULLER等[32]研究提出IL-8抑制破骨细胞吸收。IL-8是破骨细胞形成的关键调控因子,其不仅直接参与破骨细胞的形成,而且还通过刺激骨髓间充质干细胞中RANKL的表达促进破骨细胞的分化[33]。BENDRE等[8]首次报道了IL-8直接刺激破骨细胞形成和骨吸收,在培养物中添加IL-8发现成骨细胞中RANKL mRNA和蛋白质表达均上调,而骨保护素表达无明显影响;该实验向鼠类 MC3T3-E1 成骨细胞的培养物中添加IL-8 (10 ng/mL) ,结果显示RANKL mRNA表达量出现明显增幅(2.6 倍),其在6 h达到峰值,然而骨保护素水平在同一时期保持不变。此外,相同浓度的IL-8 处理可诱导 RANKL 蛋白表达。众所周知,成骨细胞表达 RANKL对于破骨细胞的形成和功能至关重要,并且先前已显示相似水平的 RANKL mRNA 表达增加支持基质细胞形成破骨细胞[34-35]。由此得出结论:添加 IL-8 增加了 RANKL mRNA 和蛋白质的表达量,从而改变了细胞中的 RANKL/骨保护素比率,使内环境向有利于破骨细胞的形成的方向发展。建立人外周血单个核细胞培养物,并在存在或不存在外源性RANKL的情况下将IL-8添加到培养物中。在这些培养物中,即使在没有外源性RANKL的情况下IL-8依旧刺激了多核细胞形成(10 d内),且由 IL-8诱导的多核细胞与用 RANKL (25 ng/mL)观察到的量是相当的。然而,当以相同浓度的IL-8和RANKL一起添加时,未观察到叠加或协同效应,表明IL-8刺激破骨细胞形成的能力不受添加物RANK-Fc 的影响,并且刺激多核细胞的能力独立于RANKL。 综上所述,向基质成骨细胞培养物中添加重组人IL-8 (10 ng/mL) 可刺激RANKL mRNA 表达和蛋白质产生,但对骨保护素的表达没有影响,并且IL-8还直接刺激人外周血单核细胞分化为破骨细胞。在这些培养物中,即使存在过量(200 ng/mL) RANK-Fc,IL-8 也能够刺激人破骨细胞的形成。这些结果证明了IL-8对破骨细胞分化和活性的可依赖RANKL途径产生间接影响,也可独立于该途径直接影响。 然而通过阻断IL-8抗体或在体外使用IL-8受体抑制剂可抑制破骨细胞的生成[36],IL-8增加RANKL mRNA和蛋白质,从而改变细胞中RANKL/骨保护素比值,促进破骨细胞形成。此外,IL-8在癌症骨转移中也起着关键作用,该过程中主要就是依靠IL-8对破骨细胞的调控来诱导骨吸收的发生,从而引起癌症的骨转移。例如在乳腺癌骨转移实验中可以观察到使用巨噬细胞集落刺激因子10A(非恶性乳腺上皮)细胞以及MDA-231(高度转移性人乳腺癌)细胞系与pSG5-COX-2载体和pEF1a-Luc-IRES-Neo载体(荧光素酶报告基因)共转染,可以检测到细胞中环氧化酶2已经过表达,并且在此基础上IL-8的产生相比正常细胞增加10-231倍不等,由此,有理由相信IL-8介导破骨细胞的产生不仅依靠前面所说的信号途径还参与环氧化酶2过表达引起的骨溶解途径[14]。但是不同的是,有学者在接种人肺癌来源细胞后形成的裸鼠的骨病变中建立人肺癌来源细胞-B细胞,与亲本人肺癌来源细胞相比,人肺癌来源细胞-B细胞中IL-8的产生减少,这表明IL-8的产生与骨转移之间可能存在关系,因此,在裸鼠的实验骨转移模型中使用转染实验性(IL-8-cDNA)和/或对照质粒的人肺癌来源细胞-B细胞检查了IL-8对骨转移的影响,2种细胞在体外的生长速率相似。对照细胞在50%的裸鼠中发生了放射学上可检测的骨转移,而实验细胞没有发生骨转移。破骨细胞骨吸收是骨转移过程中的重要步骤,IL-8对破骨细胞骨吸收具有抑制作用。这些结果表明IL-8抑制骨转移,可能是由于IL-8对破骨细胞骨吸收的抑制作用[37]。 由此可知IL-8对破骨细胞的作用可以是双向的,以往研究中多数以阻断IL-8的产生来控制破骨细胞的形成,在未来研究中可以此为切入点,探究IL-8在破骨细胞中发生拐点的深层原因,为IL-8的研究拓展新的知识面。 2.2 IL-8对血管化的作用 血管不仅将氧气和营养物质输送到骨骼,还通过调节成骨细胞、骨细胞、破骨细胞和血管细胞间的相互作用,在骨形成中发挥重要作用。近年来已发现了调控骨骼血管生成相关的细胞因子,如基质金属蛋白酶、缺氧诱导因子、结缔组织生长因子、血管内皮生长因子、成纤维细胞生长因子和Notch信号等都会影响软骨内血管的生成进而影响成骨,表明血管化与成骨之间相互关联[38]。 2.2.1 缺氧诱导因1α 缺氧诱导因子1α是介导细胞参与缺氧反应的重要转录调控因子。在缺氧环境下,缺氧诱导因子1α与缺氧诱导因子1β在细胞核中形成缺氧诱导因子1二聚体。随后该二聚体与DNA中的缺氧反应元件相结合形成三聚体,该三聚体可调控靶基因血管内皮生长因子的表达[39]。在包括骨在内的大多数组织的发育过程中,血管内皮生长因子具有良好的促血管生成作用,其由软骨细胞释放,启动血管侵入软骨,促进骨骼生长。 WANG等[40]的研究已证实,激活缺氧诱导因子1α信号通路及其下游靶点血管内皮生长因子可导致血管生成,从而影响成骨细胞的分化和活性。IL-8可促进内皮细胞迁移和血管形成[41],而在缺氧条件下可诱导人成骨细胞IL-8分泌,缺氧诱导因子1α通过直接结合相应的启动子介导缺氧诱导IL-8的表达,IL-8通过参与缺氧诱导的血管形成进而促进成骨,而这一结论已由NIU等[42]实验得以证明:发现缺氧诱导因子1α载体可以显著增强MG-63细胞中IL-6和IL-8 mRNA和蛋白的表达,在IL-8的启动子区域,存在一个功能性的缺氧诱导因子1α结合位点,可以明确得知IL-8与缺氧诱导因子1α存在互相激活和协同作用,都具有促进血管生成从而促进成骨的生物作用。但是此处IL-8与缺氧诱导因子1α之间的作用机制尚未细致说明,有待进一步研究阐明。 2.2.2 血管内皮生长因子 作为血管生成的关键调控因子之一,血管内皮生长因子在血管形成和功能发挥中都有着重要作用,是内皮细胞前体即成血管细胞在发育过程中分化和扩张所必需[43]。血管内皮生长因子家族蛋白包括血管内皮生长因子A、血管内皮生长因子B、血管内皮生长因子C、血管内皮生长因子D、血管内皮生长因子E、血管内皮生长因子F和胎盘生长因子等,其中血管内皮生长因子A作为血管生成中的主要调节因子,对骨的血管生成、骨再生成和骨修复都有极大的影响[44]。IL-8通过PI3K/AKT和MAPK/ERK信号转导通路诱导AKT、ERK磷酸化,显著增加血管内皮生长因子的生成[45]。HOU 等[45]使用大鼠模型,发现与单独骨髓间充质干细胞组相比,用IL-8预处理骨髓间充质干细胞组的治疗可减少梗死体积并增加缺血边界区血管生成,通过增强骨髓间充质干细胞中血管内皮生长因子的生成,增加了骨髓间充质干细胞的治疗潜力。这表明IL-8在MSCs中刺激血管内皮生长因子的产生可以增加MSCs重建功能微血管系统和促进恢复的能力。 2.2.3 基质金属蛋白酶 基质金属蛋白酶是一种多种锌依赖性的内肽酶,基质金属蛋白酶由结缔组织、促炎和子宫胎盘细胞分泌,包括巨噬细胞、中性粒细胞、淋巴细胞、成纤维细胞、成骨细胞、内皮细胞、血管平滑肌细胞和细胞滋养层[46],为主要降解细胞外基质的一种酶家族。基质金属蛋白酶根据其作用底物特异性分为5大类:膜型、胶原酶、基质溶血素、凝胶酶和基质溶血素,除此之外,基质金属蛋白酶还可以作用于促炎细胞因子、趋化因子和其他蛋白质等非细胞外基质底物,如IL-8、转化生长因子β、肿瘤坏死因子α,以调节炎症和免疫力的各个方面。在血管生成方面,基质金属蛋白酶可凭借其蛋白水解活性介导多种促血管生长因子的分泌促进血管生长,加快局部血管化进程[47]。同时,有学者发现基质金属蛋白酶和IL-8在血管生成时表达均显示为一致的升高,对于血管再生体现为协同作用[48],进一步研究发现IL-8特异性mRNA蛋白质表达增加可明显上调基质金属蛋白酶9 mRNA的表达活性进一步推进血管化进程[49],详见图4。"
| [1] TAKAHASHI A, DE ANDRES MC, HASHIMOTO K, et al. Epigenetic regulation of interleukin-8, an inflammatory chemokine, in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1946-1954. [2] LIN D, CHAI Y, MA Y, et al. Rapid initiation of guided bone regeneration driven by spatiotemporal delivery of IL-8 and BMP-2 from hierarchical MBG-based scaffold. Biomaterials. 2019;196:122-137. [3] RUSSO RC, GARCIA CC, TEIXEIRA MM, et al. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10(5):593-619. [4] HONG L, SHARP T, KHORSAND B, et al. MicroRNA-200c Represses IL-6, IL-8, and CCL-5 Expression and Enhances Osteogenic Differentiation. PLoS One. 2016;11(8): e0160915. [5] LIU P, LEE S, KNOLL J, et al. Loss of menin in osteoblast lineage affects osteocyte-osteoclast crosstalk causing osteoporosis. Cell Death Differ. 2017;24(4):672-682. [6] CHEN X, WANG Z, DUAN N, et al. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018;59(2):99-107. [7] KIM JM, LIN C, STAVRE Z, et al. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells. 2020;9(9):2073. [8] BENDRE MS, MONTAGUE DC, PEERY T, et al. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33(1):28-37. [9] YOSHIMURA T. Discovery of IL-8/CXCL8 (The Story from Frederick). Front Immunol. 2015;6:278. [10] KIM SJ, UEHARA H, KARASHIMA T, et al. Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia. 2001;3(1):33-42. [11] YANG A, LU Y, XING J, et al. IL-8 Enhances Therapeutic Effects of BMSCs on Bone Regeneration via CXCR2-Mediated PI3k/Akt Signaling Pathway. Cell Physiol Biochem. 2018;48(1):361-370. [12] LI A, DUBEY S, VARNEY ML, et al. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170(6):3369-3376. [13] LIU MM, DONG R, HUA Z, et al. Therapeutic potential of Liuwei Dihuang pill against KDM7A and Wnt/β-catenin signaling pathway in diabetic nephropathy-related osteoporosis. Biosci Rep. 2020;40(9):BSR20201778. [14] SINGH B, BERRY JA, VINCENT LE, et al. Involvement of IL-8 in COX-2-mediated bone metastases from breast cancer. J Surg Res. 2006;134(1):44-51. [15] WANG L, LI Y, ZHANG X, et al. Paracrine interleukin-8 affects mesenchymal stem cells through the Akt pathway and enhances human umbilical vein endothelial cell proliferation and migration. Biosci Rep. 2021;41(5):BSR20210198. [16] GUO Y, ZANG Y, LV L, et al. IL‑8 promotes proliferation and inhibition of apoptosis via STAT3/AKT/NF‑κB pathway in prostate cancer. Mol Med Rep. 2017;16(6):9035-9042. [17] WEN J, ZHAO Z, HUANG L, et al. IL-8 promotes cell migration through regulating EMT by activating the Wnt/β-catenin pathway in ovarian cancer. J Cell Mol Med. 2020;24(2):1588-1598. [18] TOOSI S, BEHRAVAN N, BEHRAVAN J. Nonunion fractures, mesenchymal stem cells and bone tissue engineering. J Biomed Mater Res A. 2018;106(9):2552-2562. [19] CIUFFI S, ZONEFRATI R, BRANDI ML. Adipose stem cells for bone tissue repair. Clin Cases Miner Bone Metab. 2017;14(2):217-226. [20] LIU H, LI D, ZHANG Y, et al. Inflammation, mesenchymal stem cells and bone regeneration. Histochem Cell Biol. 2018;149(4):393-404. [21] KANG KW, LEE SJ, KIM JH, et al. Etoposide-mediated interleukin-8 secretion from bone marrow stromal cells induces hematopoietic stem cell mobilization. BMC Cancer. 2020;20(1):619. [22] BRITSCHGI A, RADIMERSKI T, BENTIRES-ALJ M. Targeting PI3K, HER2 and the IL-8/JAK2 axis in metastatic breast cancer: Which combination makes the whole greater than the sum of its parts? Drug Resist Updat. 2013;16(3-5):68-72. [23] DOWNWARD J. PI3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15(2): 177-182. [24] SHEN L, ZHANG S, ZHANG X, et al. Enhancing the ability of autophagy and proliferation of bone marrow mesenchymal stem cells by interleukin-8 through Akt-STAT3 pathway in hypoxic environment. Sheng Wu Gong Cheng Xue Bao. 2016; 32(10):1422-1432. [25] UCCELLI A, MORETTA L, PISTOIA V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726-736. [26] PONZETTI M, RUCCI N. Osteoblast Differentiation and Signaling: Established Concepts and Emerging Topics. Int J Mol Sci. 2021;22(13):6651. [27] NIU X, CHEN Y, QI L, et al. Hypoxia regulates angeogenic-osteogenic coupling process via up-regulating IL-6 and IL-8 in human osteoblastic cells through hypoxia-inducible factor-1α pathway. Cytokine. 2019;113:117-127. [28] DA CRUZ MB, MARQUES JF, FERNANDES BF, et al. Laser surface treatment on Yttria-stabilized zirconia dental implants: Influence on cell behavior. J Biomed Mater Res B Appl Biomater. 2022;110(1):249-258. [29] CAI L, LIN D, CHAI Y, et al. MBG scaffolds containing chitosan microspheres for binary delivery of IL-8 and BMP-2 for bone regeneration. J Mater Chem B. 2018;6(27): 4453-4465. [30] AMARASEKARA DS, YUN H, KIM S, et al. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018;18(1):e8. [31] HENRIKSEN K, NEUTZSKY-WULFF AV, BONEWALD LF, et al. Local communication on and within bone controls bone remodeling. Bone. 2009;44(6):1026-1033. [32] FULLER K, OWENS JM, CHAMBERS TJ. Macrophage inflammatory protein-1 alpha and IL-8 stimulate the motility but suppress the resorption of isolated rat osteoclasts. J Immunol. 1995;154(11):6065-6072. [33] LIU X, CHEN Z, LAN T, et al. Upregulation of interleukin-8 and activin A induces osteoclastogenesis in ameloblastoma. Int J Mol Med. 2019;43(6):2329-2340. [34] THEILL LE, BOYLE WJ, PENNINGER JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795-823. [35] QUINN JM, ITOH K, UDAGAWA N, et al. Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions. J Bone Miner Res. 2001; 16(10):1787-1794. [36] KOPESKY P, TIEDEMANN K, ALKEKHIA D, et al. Millard B, Schoeberl B, Komarova SV. Autocrine signaling is a key regulatory element during osteoclastogenesis. Biol Open. 2014;3(8):767-776. [37] IGUCHI H, ONO M, MATSUSHIMA K, et al. Overproduction of IL-8 results in suppression of bone metastasis by lung cancer cells in vivo. Int J Oncol. 2000;17(2): 329-333. [38] SIVARAJ KK, ADAMS RH. Blood vessel formation and function in bone. Development. 2016;143(15):2706-2715. [39] DENG W, FENG X, LI X, et al. Hypoxia-inducible factor 1 in autoimmune diseases. Cell Immunol. 2016;303:7-15. [40] WANG Y, WAN C, DENG L, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007; 117(6):1616-1626. [41] WANG J, WANG Y, WANG S, et al. Bone marrow-derived mesenchymal stem cell-secreted IL-8 promotes the angiogenesis and growth of coloretal cancer. Oncotarget. 2015;6(40):42825-42837. [42] NIU X, CHEN Y, QI L, et al. Hypoxia regulates angeogenic-osteogenic coupling process via up-regulating IL-6 and IL-8 in human osteoblastic cells through hypoxia-inducible factor-1alpha pathway. Cytokine. 2019;113:117-127. [43] BAUTCH VL. VEGF-directed blood vessel patterning: from cells to organism. Cold Spring Harb Perspect Med. 2012;2(9):a006452. [44] HU K, OLSEN BR. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30-38. [45] HOU Y, RYU CH, JUN JA, et al. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol Int. 2014;38(9):1050-1059. [46] SAITO S, TROVATO MJ, YOU R, et al. Role of matrix metalloproteinases 1, 2, and 9 and tissue inhibitor of matrix metalloproteinase-1 in chronic venous insufficiency. J Vasc Surg. 2001;34(5):930-938. [47] WANG X, KHALIL RA. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv Pharmacol. 2018;81:241-330. [48] CHOU CH, HO CM, LAI S, et al. B-Cell Activating Factor Enhances Hepatocyte-Driven Angiogenesis via B-Cell CLL/Lymphoma 10/Nuclear Factor-KappaB Signaling during Liver Regeneration. Int J Mol Sci. 2019;20(20):5022. [49] INOUE K, SLATON JW, EVE BY, et al. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000; 6(5):2104-2119. [50] RAIMONDO S, SAIEVA L, VICARIO E, et al. Multiple myeloma-derived exosomes are enriched of amphiregulin (AREG) and activate the epidermal growth factor pathway in the bone microenvironment leading to osteoclastogenesis. J Hematol Oncol. 2019;12(1):2. [51] ZHANG Y, ZOU B, TAN Y, et al. Sinomenine inhibits osteolysis in breast cancer by reducing IL-8/CXCR1 and c-Fos/NFATc1 signaling. Pharmacol Res. 2019;142:140-150. |
| [1] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [2] | Chen Kaijia, Liu Jingyun, Cao Ning, Sun Jianbo, Zhou Yan, Mei Jianguo, Ren Qiang. Application and prospect of tissue engineering in treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1450-1456. |
| [3] | Huang Haoran, Fan Yinuo, Wei-Yang Wenxiang, Jiang Mengyu, Fang Hanjun, Wang Haibin, Chen Zhenqiu, Liu Yuhao, Zhou Chi. Urolithin A mediates p38/MAPK pathway to inhibit osteoclast activity [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1149-1154. |
| [4] | Liu Jianhong, Liao Shijie, Li Boxiang, Tang Shengping, Wei Zhendi, Ding Xiaofei. Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1076-1082. |
| [5] | Pan Xiaolong, Fan Feiyan, Ying Chunmiao, Liu Feixiang, Zhang Yunke. Effect and mechanism of traditional Chinese medicine on inhibiting the aging of mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1091-1098. |
| [6] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [7] | Feng Ruiqin, Han Na, Zhang Meng, Gu Xinyi, Zhang Fengshi. Combination of 1% platelet-rich plasma and bone marrow mesenchymal stem cells improves the recovery of peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 985-992. |
| [8] | Wang Wen, Zheng Pengpeng, Meng Haohao, Liu Hao, Yuan Changyong. Overexpression of Sema3A promotes osteogenic differentiation of dental pulp stem cells and MC3T3-E1 [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 993-999. |
| [9] | Qiu Xiaoyan, Li Bixin, Li Jingdi, Fan Chuiqin, Ma Lian, Wang Hongwu. Differentiation of insulin-producing cells from human umbilical cord mesenchymal stem cells infected by MAFA-PDX1 overexpressed lentivirus [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1000-1006. |
| [10] | Liu Qiwei, Zhang Junhui, Yang Yuan, Wang Jinjuan. Role and mechanism of umbilical cord mesenchymal stem cells on polycystic ovary syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1015-1020. |
| [11] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [12] | Wei Yuanxun, Chen Feng, Lin Zonghan, Zhang Chi, Pan Chengzhen, Wei Zongbo. The mechanism of Notch signaling pathway in osteoporosis and its prevention and treatment with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 587-593. |
| [13] | Lin Feng, Cheng Ling, Gao Yong, Zhou Jianye, Shang Qingqing. Hyaluronic acid hydrogel-encapsulated bone marrow mesenchymal stem cells promote cardiac function in myocardial infarction rats (III) [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 355-359. |
| [14] | Zhu Zhiqi, Yuan Sijie, Zhang Zilin, Ji Shijie, Meng Mingsong, Yan Anming, Han Jing. Mechanism underlying the effect of Liuwei Dihuang Pill on osteolysis and osteogenesis induced by titanium particles [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 392-397. |
| [15] | Bi Yujie, Ma Dujun, Peng Liping, Zhou Ziqiong, Zhao Jing, Zhu Houjun, Zhong Qiuhui, Yang Yuxin. Strategy and significance of Chinese medicine combined with medical hydrogel for disease treatment [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 419-425. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||