Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (21): 3393-3399.doi: 10.12307/2024.065
Previous Articles Next Articles
Novel programmed cell death in periprosthetic osteolysis
Liang Xiaolong, Zheng Kai, Geng Dechun, Xu Yaozeng
- Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215008, Jiangsu Province, China
-
Received:2023-03-04Accepted:2023-05-19Online:2024-07-28Published:2023-09-28 -
Contact:Xu Yaozeng, MD, Chief physician, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215008, Jiangsu Province, China -
About author:Liang Xiaolong, Master candidate, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215008, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. 81873991 (to GDC)
CLC Number:
Cite this article
Liang Xiaolong, Zheng Kai, Geng Dechun, Xu Yaozeng. Novel programmed cell death in periprosthetic osteolysis[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(21): 3393-3399.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
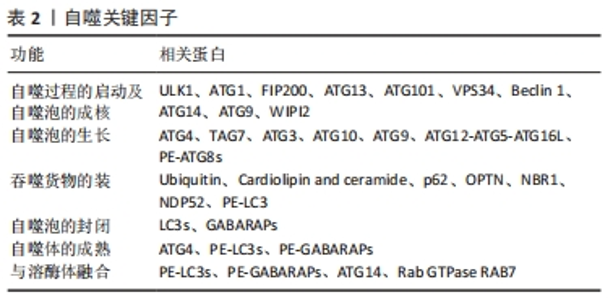
2.1 自噬 细胞自吞噬现象最早由Duve于1963年发现,镜下可见细胞内出现大量泡状结构,即双层膜吞噬泡,吞噬泡内部为细胞胞质及细胞器成分。由于早期对程序性细胞死亡的初步认识,基于上述形态学上的特点,自噬被划分为了Ⅱ类程序性细胞死亡。然而,随着时间的推移和研究的深入,人们发现自噬并非一种严格意义上的细胞死亡方式,而是一种为了应对不同形式的应激而发生的适应性过程,包括营养剥夺、生长因子耗竭、感染和缺氧[13],以保护细胞免于将要死亡的结局。本质上,自噬可作为一个细胞内动态的回收循环系统,通过自噬泡形成将胞质内物质递送到溶酶体进行降解,并为细胞更新和体内平衡产生新的代谢产物和能量[14]。因此,适度的自噬可以作为细胞的一种自我保护机制,帮助细胞应对各种复杂的环境刺激并存活,然而,过度的自噬也会适得其反,导致细胞被自身的溶酶体降解,称之为自噬性细胞死亡[15]。自噬相关基因家族是自噬体形成和自噬溶酶体递送所必需的核心蛋白,目前已研究发现了数十种自噬相关基因分子参与自噬的各个环节(表2)。此外,自噬也受到多种信号通路的调节,其中磷脂酰肌醇-3激酶对于早期吞噬泡的形成至关重要[16],而哺乳动物雷帕霉素靶蛋白则对吞噬泡的形成及成熟起抑制作用[17]。"
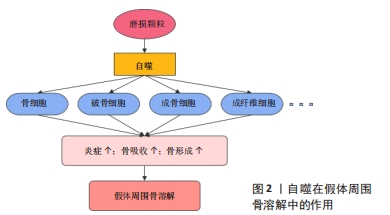
自噬已被证明与阿尔茨海默病、帕金森病、动脉粥样硬化、慢性阻塞性肺病、急性肾损伤、肝硬化、糖尿病以及癌症等多种疾病有关,而在骨骼疾病中,由于其对成骨细胞、破骨细胞及软骨细胞分化和功能的影响,自噬在骨关节炎、骨质疏松、骨佩吉特病等疾病中也发挥了重要的作用[18]。蔡艳等[19]在聚甲基丙烯酸甲酯颗粒诱导的小鼠颅骨骨溶解模型中发现自噬关键因子LC3和Beclin1的表达升高,首次证明了自噬参与了假体周围骨溶解的形成。骨细胞是骨组织中的主要细胞成分,严嘉琦等[20]使用磷酸三钙磨损颗粒成功诱导了骨溶解并发现了骨细胞自噬水平的上调,而自噬特异性抑制剂3-MA的使用增加了骨细胞的凋亡,促进了假体周围骨细胞的损伤。破骨细胞作为唯一的骨吸收细胞,在假体周围骨溶解中扮演者关键角色。体内外实验中发现,钛颗粒通过PI3K/Akt和ERK1/2信号通路促进了巨噬细胞的自噬[21-22],使得破骨细胞的数量和功能显著增加,而敲低Atg5或使用自噬抑制剂3-MA和LY294002后均可抑制破骨细胞的形成并降低破骨功能,表明抑制自噬可能是预防和治疗假体周围骨溶解的一种潜在策略[23-24]。CHU等[25]发现泽兰黄酮可通过抑制核因子κB和丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号通路及肿瘤坏死因子受体相关因子6介导的Beclin1泛素化途径,降低破骨细胞自噬的激活,有效减轻钛颗粒诱导的骨吸收和骨质破坏。最新的研究成果表明,成骨细胞在假体周围骨溶解中也发挥着重要的作用。多项研究发现,磨损颗粒显著上调了成骨细胞中自噬相关蛋白Atg5、LC3和Beclin1的表达,重楼皂苷Ⅰ可减轻磷酸三钙磨损颗粒诱导的成骨细胞自噬来增强成骨功能[26],金丝桃苷通过P38/MAPK通路抑制自噬保护成骨细胞免受钛颗粒诱导的损伤[27],而铝纳米颗粒和泛素蛋白酶体抑制剂可通过抑制核因子κB途径减弱自噬,从而减轻炎症并促进成骨功能[28]。此外,钛颗粒还诱导增强了成纤维细胞的自噬并增加了CX3CL1的表达,促进了局部单核细胞的募集和炎症,进一步加重假体周围骨溶解[29]。相反,另一研究表明,Al2O3颗粒诱导的成纤维细胞自噬减弱了核因子κB受体活化因子配体的表达,降低自噬水平则进一步促进了骨溶解[30]。 总的来说,自噬在假体周围骨溶解有着很重要的影响,多种磨损颗粒均可以提高骨细胞、破骨细胞、成骨细胞和成纤维细胞等自噬的水平,这可能是细胞对于磨损颗粒刺激的一种自我保护,但过度的自噬最终加重了局部炎症,打破了骨形成和骨吸收的代谢平衡,进一步促进骨溶解(图2)。一方面,通过抑制自噬可以有效逆转磨损颗粒诱导的骨溶解,而另一方面,自噬的过度抑制也可能不利于细胞的存活并促进细胞凋亡,因此,如何适度的调节自噬水平可能是治疗假体周围骨溶解的一种潜在策略。"
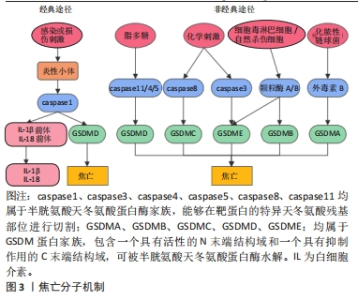
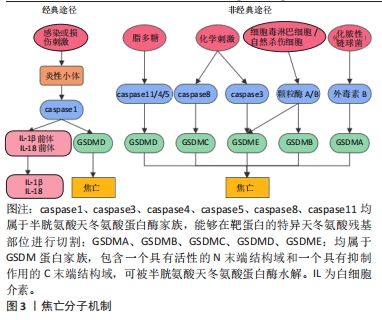
2.2 焦亡 2001年COOKSON等[31]正式将感染鼠伤寒沙门氏菌的巨噬细胞中发生的促炎性细胞死亡方式定义为“焦亡”,是一种由炎性小体引发并由Gasdermin D(GSDMD)蛋白介导的细胞程序性死亡。根据形态上的特征,焦亡被归类为Ⅲ型程序性细胞死亡,表现为细胞的肿胀,继而发生细胞膜的穿孔或破裂,并释放大量炎症因子,而与细胞凋亡中表现的细胞核破坏不同,发生焦亡的细胞细胞核通常保持完整[32]。在分子机制上,炎性小体的形成是焦亡发生的起始步骤,主要由3个组分构成:富含亮氨酸重复序列的NOD样受体,含有半胱天冬酶募集结构域的凋亡相关斑点样蛋白以及caspase的前体蛋白。根据核心蛋白的不同,炎性小体主要包括NOD样受体蛋白1(Nod-like receptor protein 1,NLRP1)、NLRP3、NLRC4、AIM2和Pyrin等。此外,Gasdermin(GSDM)蛋白家族在细胞焦亡中起关键作用,主要包括GSDMA、GSDMB、GSDMC、 GSDMD、GSDME和PJVK[33]。根据焦亡过程中所依赖的caspase蛋白的不同,细胞焦亡又分为经典途径和非经典途径(图3)。在经典途径中,模式识别受体通过识别病原体相关分子模式和损伤相关分子模式,启动炎性小体的组装,继而招募并活化caspase1,使其切割GSDMD产生具有活性的N端和C端结构域,其中GSDMD-N可嵌插入细胞膜脂质形成膜孔,导致细胞渗透压破坏、肿胀、破裂并引发焦亡;同时,活化的caspase1还作用于白细胞介素1β、白细胞介素18前体物质,介导白细胞介素1β、白细胞介素18的大量释放,因此具有强大的促炎作用。而在不依赖caspase1的非经典途径中,caspase11/4/5可以直接被脂多糖等激活,切割GSDMD介导细胞焦亡[34]。此外,在特定的化学刺激下,caspase8和caspase3可分别切割GSDMC和GSDME诱导焦亡[35-36],细胞毒淋巴细胞和自然杀伤细胞释放的颗粒酶A/B可分别裂解GSDMB和GSDME促进焦亡,而化脓性链球菌分泌的外毒素B可特异性切割GSDMA来引发焦亡。"
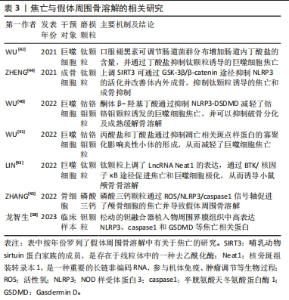
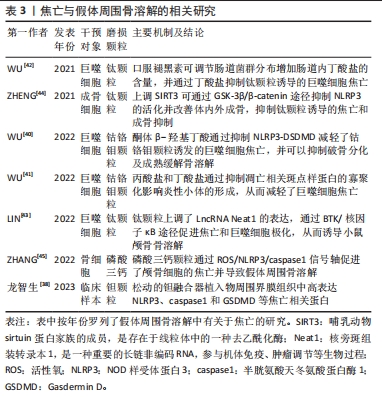
由于焦亡独特的促炎作用,其在各种炎症性疾病中起关键作用,包括心肌炎、慢性阻塞性肺疾病以及各种癌症等等。NLRP3也被证实主要在巨噬细胞/破骨细胞、成骨细胞、树突状细胞、中性粒细胞和淋巴细胞等中表达[37]。对发生钽融合器松动的临床样本组织进行分析后发现,植入物的周围存在大量金属颗粒并伴随着纤维组织增生,且植入物周围界膜组织中NLRP3、caspase1和GSDMD表达均显著升高,说明了细胞焦亡也参与了假体周围骨溶解的发生发展[38]。有关假体周围骨溶解中细胞焦亡的相关研究,见表3。目前认为,巨噬细胞由于吞噬各种磨损颗粒导致了溶酶体破裂和蛋白酶组织蛋白酶B的渗漏,继而激活NLRP3炎性小体并激活细胞焦亡,促进骨溶解[39]。WU等[40]发现,钴铬钼颗粒显著上调了巨噬细胞内凋亡相关斑点样蛋白和caspase1的表达,并引发下游白细胞介素1β和白细胞介素18的大量释放,而酮体β-羟基丁酸可通过抑制NLRP3-DSDMD途径来缓解钴铬钼颗粒诱发的焦亡,此外,酮体β-羟基丁酸还降低了细胞内TRAF6和NFATc1的表达,降低了基质金属蛋白酶9、CTSK和TRAP的水平,抑制了巨噬细胞的破骨分化及骨吸收能力。同一研究小组的另一项实验表明,丙酸盐和丁酸盐还可通过阻碍巨噬细胞中凋亡相关斑点样蛋白寡聚化来抑制NLRP3活化,从而抑制焦亡的进一步发生[41]。基于上述发现,该团队还通过给予褪黑素调节肠道中某些产生短链脂肪酸细菌的相对丰度,以增加肠道丁酸盐的产量,进而缓解了钛颗粒诱导的小鼠颅骨骨溶解[42]。另一项研究表明,布鲁顿酪氨酸激酶BTK可促进凋亡相关斑点样蛋白的寡聚化和caspase1的活化,而钛颗粒可以上调其上游长联非编码RNA Neat1在巨噬细胞中的表达,通过si-Neat1可显著抑制核因子κB通路和NLRP3的活化,以逆转假体周围骨溶解的发生[43]。另一方面,成骨细胞在假体周围骨溶解的作用也十分重要。在钛颗粒诱导的大鼠股骨骨溶解模型中,ZHENG等[44]发现SIRT3的消耗促进了NLRP3的激活,而靶向上调SIRT3显著抑制了NLRP3活化及caspase1、GSDMD、白细胞介素1β、白细胞介素18的表达,并通过Wnt/β-catenin信号通路促进成骨功能,有效减轻了钛颗粒诱导的骨溶解。除此之外,磷酸三钙诱导的小鼠颅骨骨溶解模型表明,磷酸三钙磨损颗粒也上调了焦亡相关分子的表达水平,并证明了活性氧是激活NLRP3炎性小体的关键因素之一,活性氧清除剂的使用则抑制了焦亡及骨溶解的发生[45]。"
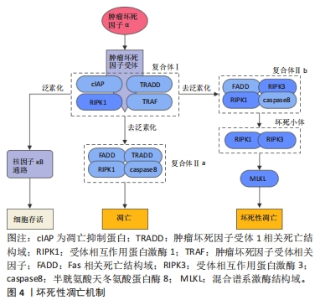
2.3 坏死性凋亡 坏死性凋亡是一种非半胱天冬酶依赖性的细胞程序性死亡方式,与坏死具有相似的形态特征,表现为胞质和细胞器肿胀、胞膜破裂以及细胞内容物的渗漏[46]。分子机制上,坏死性凋亡可由多种死亡受体配体诱导,包括肿瘤坏死因子超家族受体、Toll样受体、干扰素受体等,并由受体相互作用蛋白激酶1(receptor interacting protein kinase 1,RIPK1)、RIPK3和混合谱系激酶结构域介导。其中肿瘤坏死因子诱导的坏死性凋亡途径被研究的最为深入(图4)。当肿瘤坏死因子α与细胞表面的肿瘤坏死因子受体1结合后,触发肿瘤坏死因子受体1相关死亡结构域、RIPK1、凋亡抑制蛋白、肿瘤坏死因子受体相关因子等蛋白的募集并形成复合物Ⅰ,复合物Ⅰ可通过复杂的模式变化协调下游信号通路,从而决定细胞的存活或死亡。正常情况下,复合物Ⅰ中的RIPK1可经历泛素化修饰募集转化生长因子β活化激酶1、转化生长因子β活化激酶1结合蛋白2和转化生长因子β活化激酶1结合蛋白3,进一步激活核因子κB信号通路并促进细胞的存活,而当RIPK1被去泛素化修饰时,RIPK1可被释放出来并参与复合物Ⅱ的形成,而复合物Ⅱ的类型和caspase8的状态将决定细胞向凋亡或坏死性凋亡的转变[47]。其中RIPK1、TRADD、Fas相关死亡结构域和caspase8可形成复合物Ⅱa,促使细胞发生凋亡,而caspase8被抑制后,RIPK1、RIPK3、FADD和caspase8组成的复合物Ⅱb可诱导细胞发生坏死性凋亡[48]。随着复合物Ⅱb的形成,RIPK1可发生自磷酸化被激活,并通过其RIP同型结构域与RIPK3相互作用形成RIPK1/RIPK3二聚体,即坏死小体,随后坏死小体中磷酸化的RIPK3可作用于其功能底物混合谱系激酶结构域,促进混合谱系激酶结构域寡聚化并转移到质膜上,从而引起质膜透化和完整性丧失,发生细胞坏死性凋亡[49]。这一过程可以被Nec-1靶向与RIPK1相互作用从而特异性抑制坏死性凋亡[49]。总之,RIPK1的磷酸化对于坏死小体的组装和激活至关重要,而RIPK3则是坏死性凋亡不可或缺的关键因子,决定了细胞发生坏死性凋亡的易感性[50],并最终由混合谱系激酶结构域执行。"
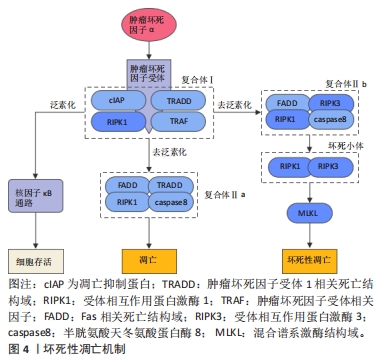
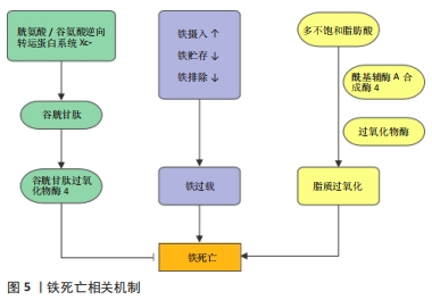
肿瘤坏死因子α是诱导细胞坏死性凋亡的主要细胞因子,临床研究发现,在全髋关节置换术后发生了假体周围骨溶解的患者相较于对照组,血清中肿瘤坏死因子α的水平显著增高[51],而抗肿瘤坏死因子α疗法也是目前临床防治假体周围骨溶解最有潜力的方法之一[52]。尽管如此,在假体周围骨溶解中是否发生了坏死性凋亡以及其具体作用机制有待进一步研究。在体外实验中发现,肿瘤坏死因子α诱导的成骨细胞死亡同时存在凋亡和坏死性凋亡,并可以相互转变,同时应用肿瘤坏死因子α和caspasr8特异性抑制剂Z-IETD-FMK促使细胞发生坏死性凋亡,并可被Nec-1逆转[53]。在破骨细胞的研究中发现,抑制RIPK1泛素化从而激活RIPK1诱导坏死性凋亡后,人破骨细胞表现出较低的稳定性和活力,表明坏死性凋亡抑制了破骨细胞的形成[54]。此外,在RIPK3缺陷小鼠中,观察到骨干小梁化程度急剧降低,抗酒石酸酸性磷酸酶染色的骨切片结果也表明,RIPK3缺陷动物骨骼中的破骨细胞总数显著增加[55]。上述研究表明,坏死性凋亡可以降低破骨细胞的数目和功能,抑制骨吸收,相反,成骨细胞的坏死性凋亡则会导致骨形成减少。因此,进一步研究阐明坏死性凋亡在假体周围骨溶解中的作用及机制,可以为防治假体周围骨溶解提供新的策略。 2.4 铁死亡 铁死亡是一种新型的细胞程序性死亡方式,于2012年由Dixon团队正式提出并命名,其特征在于铁依赖性脂质过氧化的积累。在形态学表现上,不同于细胞凋亡和其他形式的细胞程序性死亡,铁死亡以线粒体表现为主,体现为线粒体明显萎缩,膜密度增加和线粒体嵴的减少或消失[56]。铁死亡的发生与铁代谢和脂质调节密切相关,而对于铁死亡的调控机制(图5),目前主要分为3类:①谷胱甘肽和谷胱甘肽过氧化物酶4(glutathione peroxidase 4,GPX4)途径:GPX4是铁死亡的关键调节蛋白,通过将谷胱甘肽氧化使得细胞毒性脂质过氧化物还原为相应的醇,以防止脂质过氧化的发生[57],GPX4活性的抑制将导致脂质过氧化物的蓄积从而诱导铁死亡。由SLC7A11和SLC3A2两个亚基组成的胱氨酸/谷氨酸逆向转运蛋白系统Xc-负责从胞外摄入胱氨酸,胱氨酸在胞内被还原为半胱氨酸并参与谷胱甘肽的合成[56],任何因素引起的谷胱甘肽合成降低也将影响GPX4的活性,导致细胞抗氧化能力降低和铁死亡。②铁代谢调控途径:铁是体内重要的微量元素,在氧的运输、电子传递、DNA合成等过程中发挥重要作用。正常生理情况下,Fe3+通过转铁蛋白进入细胞并被金属还原酶STEAP3还原为Fe2+,并主要以铁蛋白形式贮存,只有一小部分游离Fe2+发挥作用,多余的Fe2+可经过铜蓝蛋白氧化形成Fe3+后再次通过转铁蛋白转运至胞外从而维持细胞内的铁稳态。当铁代谢过程发生紊乱时,如铁摄入的增加、铁蛋白的过度降解、铁排出的抑制等都将导致胞内游离铁离子蓄积,继而通过芬顿反应产生大量羟自由基,导致膜磷脂发生损伤,并最终引起细胞铁死亡[58-59]。③脂质代谢调节途径:脂质过氧化是铁死亡的标志,涉及复杂的脂质代谢过程,其中酰基辅酶A合成酶4介导长链脂肪酸和辅酶A的酯化反应,是决定铁死亡敏感性的关键因素。酰基辅酶A合成酶4将花生四烯酸等多不饱和脂肪酸转化为脂酰辅酶A,并将其掺入细胞膜脂质[60]。随后,多种过氧化物酶如脂氧合酶、环氧合酶和细胞色素P450等介导了脂质的过氧化,由铁离子芬顿反应产生的自由基也可以导致非酶依赖性的脂质过氧化[61]。"
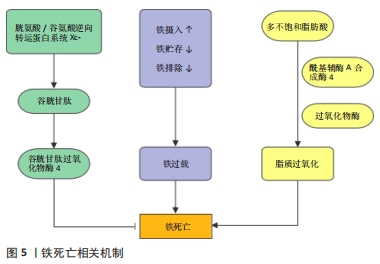

在铁死亡发现后的10年里,多种疾病或器官损伤被报道与铁死亡有关,包括Sedaghatian型脊椎干骺端发育不良、多器官功能障碍综合征、视网膜变性、神经变性、自身免疫疾病、肿瘤等等[62],在骨质疏松、骨关节炎和骨肉瘤等骨科疾病中也被证实铁死亡的存在和意义[63-65]。在假体周围骨溶解领域,铁死亡的作用及机制研究也正在成为人民日益关注的热点。通过分析临床假体周围骨溶解样本,XU等[66]发现松动假体周围骨组织成骨相关指标及Gpx4、Slc7a11的mRNA表达显著下降,提示了铁死亡的激活和成骨细胞的抑制。该团队进一步发现钴铬钼纳米颗粒可以通过下调Nrf2-ARE信号通路诱导成骨细胞发生铁死亡,表现出线粒体皱缩、线粒体嵴消失等典型电镜改变,并伴随Gpx4、Slc7a11蛋白水平上调和酰基辅酶A合成酶4、环氧合酶2的下调,同时,丙二醛作为脂质过氧化作用的最终产物,其表达在钴铬钼颗粒干预后也显著增加。此外,铁死亡抑制剂Ferrostatin‐1通过逆转成骨细胞的铁死亡,有效提高了成骨功能,明显改善了钴铬钼颗粒诱导的骨溶解。此外,其他研究还发现,细胞内铁过载不仅可以抑制成骨细胞的活性及功能[67],还可以通过上调核因子κB通路和氧化应激促进单核细胞向破骨细胞分化,刺激破骨细胞活性[68],从而导致骨稳态失衡。但是假体周围骨溶解时是否存在铁过载还需要进一步明确,对于铁死亡在假体周围骨溶解中的作用及机制还需要进一步深入研究。"

| [1] SINGH JA, YU S, CHEN L, et al. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. J Rheumatol. 2019;46(9):1134-1140. [2] BURKE NG, GIBBONS JP, CASSAR-GHEITI AJ, et al. Total hip replacement—the cause of failure in patients under 50 years old? Ir J Med Sci. 2019; 188(3):879-883. [3] CHARETTE RS, SLOAN M, DEANGELIS RD, et al. Higher Rate of Early Revision Following Primary Total Knee Arthroplasty in Patients Under Age 55: A Cautionary Tale. J Arthroplasty. 2019;34(12):2918-2924. [4] WEBER M, RENKAWITZ T, VOELLNER F, et al. Revision Surgery in Total Joint Replacement Is Cost-Intensive. Biomed Res Int. 2018;2018:8987104. [5] HUNT LP, WHITEHOUSE MR, BESWICK A, et al. Implications of Introducing New Technology: Comparative Survivorship Modeling of Metal-on-Metal Hip Replacements and Contemporary Alternatives in the National Joint Registry. J Bone Joint Surg Am. 2018;100(3):189-196. [6] CHALMERS BP, SYKU M, JOSEPH AD, et al. High Rate of Re-Revision in Patients Less Than 55 Years of Age Undergoing Aseptic Revision Total Knee Arthroplasty. J Arthroplasty. 2021;36(7):2348-2352. [7] GALLO J, GOODMAN SB, KONTTINEN YT, et al. Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms. Acta Biomater. 2013; 9(9):8046-8058. [8] GALLO J, GOODMAN SB, KONTTINEN YT, et al. Particle disease: Biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013;19(2):213-224. [9] GOODMAN SB, GALLO J. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J Clin Med. 2019;8(12):2091. [10] BEDOUI S, HEROLD MJ, STRASSER A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020;21(11):678-695. [11] 刘国印, 赵建宁, 王瑞. 磨损微粒诱导细胞凋亡与无菌性松动的研究进展[J]. 中国骨伤,2013,26(9):791-796. [12] 蒋营军, 吴连国. 人工关节置换术后磨损颗粒与假体周围骨溶解的研究进展[J]. 中国骨伤,2016,29(10):968-972. [13] DIKIC I, ELAZAR Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349-364. [14] MIZUSHIMA N, KOMATSU M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728-741. [15] NAH J, ZABLOCKI D, SADOSHIMA J. The role of autophagic cell death in cardiac disease. J Mol Cell Cardiol. 2022;173:16-24. [16] HURLEY JH, YOUNG LN. Mechanisms of Autophagy Initiation. Annual Review of Biochemistry. 2017;86(1):225-244. [17] KIM YC, GUAN KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125(1):25-32. [18] KLIONSKY DJ, PETRONI G, AMARAVADI RK, et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863. [19] 蔡燕, 施勤, 赵环, 等. 聚甲基丙烯酸甲酯颗粒诱导骨溶解实验研究[J]. 重庆医学,2013,42(34):4160-4161+4165. [20] 严嘉琦, 张云, 刘方舒, 等. 磷酸三钙磨损颗粒诱导小鼠假体周围骨细胞损伤的作用[J]. 中国应用生理学杂志,2018,34(1):83-87. [21] XIAN G, CHEN W, GU M, et al. Titanium particles induce apoptosis by promoting autophagy in macrophages via the PI3K/Akt signaling pathway. J Biomed Mater Res A. 2020;108(9):1792-1805. [22] WANG L, GAO Z, ZHANG J, et al. Netrin-1 regulates ERK1/2 signaling pathway and autophagy activation in wear particle-induced osteoclastogenesis. Cell Biol Int. 2021;45(3):612-622. [23] CHEN W, XIAN G, GU M, et al. Autophagy inhibitors 3-MA and LY294002 repress osteoclastogenesis and titanium particle-stimulated osteolysis. Biomater Sci. 2021;9(14):4922-4935. [24] CHEN J, YAO Y, WANG Y, et al. Autophagy triggered by the ROS/ERK signaling pathway protects mouse embryonic palatal cells from apoptosis induced by nicotine. Environ Sci Pollut Res Int. 2022;29(54):81909-81922. [25] CHU B, CHEN S, ZHENG X, et al. Nepetin inhibits osteoclastogenesis by inhibiting RANKL-induced activation of NF-κB and MAPK signalling pathway, and autophagy. J Cell Mol Med. 2020;24(24):14366-14380. [26] 董凡赫, 寿今豪, 陈宇峰, 等. 重楼皂苷I通过抑制自噬减轻磷酸三钙磨损颗粒诱导成骨细胞损伤的实验研究[J]. 中草药,2020,51(9):2501-2508. [27] ZHANG Q, ZHANG XF. Hyperoside decreases the apoptosis and autophagy rates of osteoblast MC3T3-E1 cells by regulating TNF-like weak inducer of apoptosis and the p38mitogen activated protein kinase pathway. Mol Med Rep. 2019;19(1):41-50. [28] ZHANG Z, FU X, XU L, et al. Nanosized Alumina Particle and Proteasome Inhibitor Bortezomib Prevented inflammation and Osteolysis Induced by Titanium Particle via Autophagy and NF-κB Signaling. Sci Rep. 2020;10(1): 5562. [29] WU W, WANG L, MAO YQ, et al. Impaired Autophagy in the Fibroblasts by Titanium Particles Increased the Release of CX3CL1 and Promoted the Chemotactic Migration of Monocytes. Inflammation. 2020;43(2):673-685. [30] LI D, WANG C, LI Z, et al. Nano-sized Al2O3 particle-induced autophagy reduces osteolysis in aseptic loosening of total hip arthroplasty by negative feedback regulation of RANKL expression in fibroblasts. Cell Death Dis. 2018; 9(8):840. [31] COOKSON BT, BRENNAN MA. Pro-inflammatory programmed cell death. Trends in Microbiology. 2001;9(3):113-114. [32] GALLUZZI L, VITALE I, AARONSON SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486-541. [33] XUE W, CUI D, QIU Y. Research Progress of Pyroptosis in Alzheimer’s Disease. Front Mol Neurosci. 2022;15:872471. [34] MAN SM, KARKI R, KANNEGANTI TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61-75. [35] NEWTON K, WICKLIFFE KE, MALTZMAN A, et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature. 2019;575(7784): 679-682. [36] WANG Y, GAO W, SHI X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99-103. [37] ZHONG Y, KINIO A, SALEH M. Functions of NOD-Like Receptors in Human Diseases. Front Immunol. 2013;4:333. [38] 龙智生, 扶流祥, 龚飞鹏, 等. 细胞焦亡相关蛋白在钽融合器松动周围组织中的表达及意义[J]. 中国组织工程研究,2023,27(25):4057-4062. [39] SON HS, LEE J, LEE HI, et al. Benzydamine inhibits osteoclast differentiation and bone resorption via down-regulation of interleukin-1β expression. Acta Pharm Sin B. 2020;10(3):462-474. [40] WU Y, TENG Y, ZHANG C, et al. The ketone body β-hydroxybutyrate alleviates CoCrMo alloy particles induced osteolysis by regulating NLRP3 inflammasome and osteoclast differentiation. J Nanobiotechnology. 2022; 20:120. [41] WU YL, ZHANG CH, TENG Y, et al. Propionate and butyrate attenuate macrophage pyroptosis and osteoclastogenesis induced by CoCrMo alloy particles. Mil Med Res. 2022;9:46. [42] WU Y, HE F, ZHANG C, et al. Melatonin alleviates titanium nanoparticles induced osteolysis via activation of butyrate/GPR109A signaling pathway. J Nanobiotechnology. 2021;19:170. [43] LIN S, WEN Z, LI S, et al. LncRNA Neat1 promotes the macrophage inflammatory response and acts as a therapeutic target in titanium particle-induced osteolysis. Acta Biomater. 2022;142:345-360. [44] ZHENG K, BAI J, LI N, et al. Protective effects of sirtuin 3 on titanium particle-induced osteogenic inhibition by regulating the NLRP3 inflammasome via the GSK-3β/β-catenin signalling pathway. Bioact Mater. 2021;6(10):3343-3357. [45] ZHANG Y, YAN M, NIU W, et al. Tricalcium phosphate particles promote pyroptotic death of calvaria osteocytes through the ROS/NLRP3/Caspase-1 signaling axis in amouse osteolysis model. Int Immunopharmacol. 2022; 107:108699. [46] GALLUZZI L, KEPP O, CHAN FKM, et al. Necroptosis: Mechanisms and Relevance to Disease. Annu Rev Pathol. 2017;12:103-130. [47] JACO I, ANNIBALDI A, LALAOUI N, et al. MK2 Phosphorylates RIPK1 to Prevent TNF-Induced Cell Death. Mol Cell. 2017;66(5):698-710.e5. [48] HU X, WANG Z, KONG C, et al. Necroptosis: A new target for prevention of osteoporosis. Front Endocrinol (Lausanne). 2022;13:1032614. [49] CAI Z, JITKAEW S, ZHAO J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16(1):55-65. [50] MOUJALLED DM, COOK WD, OKAMOTO T, et al. TNF can activate RIPK3 and cause programmed necrosis in the absence of RIPK1. Cell Death Dis. 2013;4(1):e465-e465. [51] CHAGANTI RK, PURDUE E, SCULCO TP, et al. Elevation of Serum Tumor Necrosis Factor α in Patients with Periprosthetic Osteolysis: A Case-Control Study. Clin Orthop Relat Res. 2014;472(2):584-589. [52] SCHWARZ EM, LOONEY RJ, O’KEEFE RJ. Anti-TNF-α therapy as a clinical intervention for periprosthetic osteolysis. Arthritis Res. 2000;2(3):165-168. [53] SHI G, JIA P, CHEN H, et al. Necroptosis occurs in osteoblasts during tumor necrosis factor-α stimulation and caspase-8 inhibition. Braz J Med Biol Res. 2018;52(1):e7844. [54] MOEN IN, WESTHRIN M, HÅLAND E, et al. Smac-mimetics reduce numbers and viability of human osteoclasts. Cell Death Discov. 2021;7(1):36. [55] MULLIN BH, TICKNER J, ZHU K, et al. Characterisation of genetic regulatory effects for osteoporosis risk variants in human osteoclasts. Genome Biol. 2020;21(1):80. [56] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: An Iron-Dependent Form of Non-Apoptotic Cell Death. Cell. 2012;149(5):1060-1072. [57] YANG WS, SRIRAMARATNAM R, WELSCH ME, et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell. 2014;156(1-2):317-331. [58] WANG YQ, CHANG SY, WU Q, et al. The Protective Role of Mitochondrial Ferritin on Erastin-Induced Ferroptosis. Front Aging Neurosci. 2016;8:308. [59] GENG N, SHI BJ, LI SL, et al. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci. 2018;22(12):3826-3836. [60] DOLL S, PRONETH B, TYURINA YY, et al. Acsl4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat Chem Biol. 2017;13(1): 91. [61] CLEMENTE SM, MARTÍNEZ-COSTA OH, MONSALVE M, et al. Targeting Lipid Peroxidation for Cancer Treatment. Molecules. 2020;25(21):5144. [62] STOCKWELL BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185(14):2401-2421. [63] JENEY V. Clinical Impact and Cellular Mechanisms of Iron Overload-Associated Bone Loss. Front Pharmacol. 2017;8:77. [64] JING X, DU T, LI T, et al. The detrimental effect of iron on OA chondrocytes: Importance of pro-inflammatory cytokines induced iron influx and oxidative stress. J Cell Mol Med. 2021;25(12):5671-5680. [65] NI S, KUANG Y, YUAN Y, et al. Mitochondrion-mediated iron accumulation promotes carcinogenesis and Warburg effect through reactive oxygen species in osteosarcoma. Cancer Cell Int. 2020;20(1):399. [66] XU Y, SANG W, ZHONG Y, et al. CoCrMo‐Nanoparticles induced peri‐implant osteolysis by promoting osteoblast ferroptosis via regulating Nrf2‐ARE signalling pathway. Cell Prolif. 2021;54(12):e13142. [67] DOYARD M, FATIH N, MONNIER A, et al. Iron excess limits HHIPL-2 gene expression and decreases osteoblastic activity in human MG-63 cells. Osteoporos Int. 2012;23(10):2435-2445. [68] YANG J, DONG D, LUO X, et al. Iron Overload-Induced Osteocyte Apoptosis Stimulates Osteoclast Differentiation Through Increasing Osteocytic RANKL Production In Vitro. Calcif Tissue Int. 2020;107(5):499-509. |
| [1] | Yu Weijie, Liu Aifeng, Chen Jixin, Guo Tianci, Jia Yizhen, Feng Huichuan, Yang Jialin. Advantages and application strategies of machine learning in diagnosis and treatment of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1426-1435. |
| [2] | Lin Zeyu, Xu Lin. Research progress in gout-induced bone destruction mechanism [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1295-1300. |
| [3] | Shen Jiangyong, He Xi, Tang Yuting, Wang Jianjun, Liu Jinyi, Chen Yuanyuan, Wang Xinyi, Liu Tong, Sun Haoyuan. RAS-selective lethal small molecule 3 inhibits the fibrosis of pathological scar fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1168-1173. |
| [4] | Wang Ji, Zhang Min, Li Wenbo, Yang Zhongya, Zhang Long. Effect of aerobic exercise on glycolipid metabolism, skeletal muscle inflammation and autophagy in type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1200-1205. |
| [5] | Zhou Bangyu, Li Jie, Ruan Yushang, Geng Funeng, Li Shaobo. Effects of Periplaneta americana powder on motor function and autophagic protein Beclin-1 in rats undergoing spinal cord hemisection [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1223-1228. |
| [6] | Sheng Siqi, Xie Lin, Zhao Xiangyu, Jiang Yideng, Wu Kai, Xiong Jiantuan, Yang Anning, Hao Yinju, Jiao Yun. Involvement of miR-144-3p in Cbs+/- mouse hepatocyte autophagy induced by high-methionine diet [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1289-1294. |
| [7] | Huang Yuxin, Liang Wenzi, Chen Xiuwen, Ni Na, Zhao Yinglin, Lin Changmin. Role of autophagy in hair regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1112-1117. |
| [8] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [9] | Liu Qiwei, Zhang Junhui, Yang Yuan, Wang Jinjuan. Role and mechanism of umbilical cord mesenchymal stem cells on polycystic ovary syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1015-1020. |
| [10] | Liu Tao, Zhang Wenkai, Ma Ziqian, Zhang Yan, Chen Xueming. Riluzole interferes with the activation of NLRP3 inflammasome in microglia of rats with spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1036-1042. |
| [11] | Pan Xiaolong, Fan Feiyan, Ying Chunmiao, Liu Feixiang, Zhang Yunke. Effect and mechanism of traditional Chinese medicine on inhibiting the aging of mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1091-1098. |
| [12] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [13] | Li Jiaqi, Huang Yuanli, Li Yan, Wang Chunren, Han Qianqian. Mechanism and influencing factors in molecular weight degradation of non-cross-linked hyaluronic acid [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 747-752. |
| [14] | Xu Rong, Wang Haojie, Geng Mengxiang, Meng Kai, Wang Hui, Zhang Keqin, Zhao Huijing. Research advance in preparation and functional modification of porous polytetrafluoroethylene artificial blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 759-765. |
| [15] | Chen Xiaofang, Zheng Guoshuang, Li Maoyuan, Yu Weiting. Preparation and application of injectable sodium alginate hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 789-794. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||