Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (36): 5855-5861.doi: 10.3969/j.issn.2095-4344.2017.36.020
Previous Articles Next Articles
Coupling of osteogenesis and angiogenesis in bones
Yang Jin-ting, Han Xiang-long
- State Key Laboratory for Oral Diseases, Department of Orthodontics, West China School of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
-
Received:2017-09-23Online:2017-12-28Published:2018-01-04 -
Contact:Han Xiang-long, M.D., Associate professor, Master’s supervisor, State Key Laboratory for Oral Diseases, Department of Orthodontics, West China School of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
About author:Yang Jin-ting, Master, State Key Laboratory for Oral Diseases, Department of Orthodontics, West China School of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
Supported by:the National Natural Science Foundation of China,No. 81371172 and 81671024
CLC Number:
Cite this article
Yang Jin-ting, Han Xiang-long. Coupling of osteogenesis and angiogenesis in bones[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(36): 5855-5861.
share this article
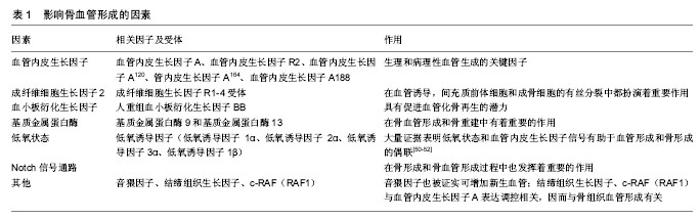
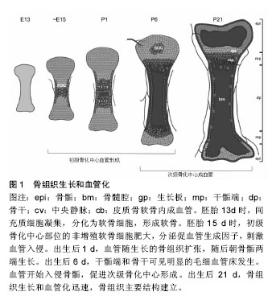
2.1 血管及血管形成 血管是生物运送血液、营养、氧、代谢产物、细胞因子以及细胞源等的管道,由几种不同的细胞构成,内层为内皮细胞,周围覆盖管周细胞(或称壁细胞),这些壁细胞又根据其形态及表面标志物的不同,被分为周细胞和血管平滑肌细胞。周细胞嵌入内皮细胞基底膜下,与毛细血管内皮细胞发生直接接触;血管平滑肌细胞主要覆盖较大的血管,静脉和动脉,与内皮细胞没有直接接触[2]。 血管的新生包括血管发生和血管形成两个过程。早期胚胎发育中,中胚层细胞祖细胞分化出的血管内皮祖细胞经募集、增殖、分化和迁移,形成功能尚不成熟的初级血管丛,此过程称为血管发生。而血管形成是指已存在的血管网的扩张延展,微血管以出芽或分裂的方式从已存在的血管床长出,并形成新的血管分支及毛细血管丛,这一系列过程包括内皮细胞出芽、迁移、增殖、管腔吻合和血管修整[3-6]。血管形成的实现要求不同血管细胞间的广泛性协调,保证新生血管具备完全的功能和稳定。例如,毛细血管床的扩张过程涉及内皮细胞向动、静脉方向特异性分化,使其形成动脉或静脉。周细胞和平滑肌细胞在血管重塑、稳定、成熟中也是必须的[6-7]。越来越多的证据表明血管形成和器官特异性分化,受到局部微环境的调控,形成特异性的内皮细胞[8-10]。这种特异性分化的机制也体现在骨组织的血管结构中,下文将进一步阐述。 2.2 骨组织的血管结构 骨骼系统是一个适应性反馈调控系统,与机械信号、生化信号、神经信号持续性整 合[11]。高度钙化,基质丰富的骨组织,骨髓中含有造血细胞,其功能不仅涉及钙磷代谢和转移,还与能量代谢和造血生成相关,并且各功能间存在相互联系。对一些功能来说,这些联系的基本要素就是骨血管化。骨作为一个高度血管化的组织,又存在一些与其他器官血管不一致的地方如生长板和关节软骨。和其他器官一样,骨组织中的血管系统表现出典型的层次结构,动脉干分支流入广泛的毛细血管网,然后汇入骨干中心的大静脉。骨干是骨含骨髓的主要部位[12]。 基于标志物表达和功能特征,骨组织的毛细血管可分为H和L两个亚类[12],而H型和L型毛细血管相互交联。H型毛细血管在骨骺端,即无血管生长板区域。骨骺端H型毛细血管呈管束状排列,在远端近生长板的区域相互交联。此外,H型毛细血管还分布在骨干段靠近密质骨的骨内膜区域。H型毛细血管与血管周围表达Osterix的骨祖细胞相联系,且高表达连接蛋白CD31(或PECAM1)和唾液酸糖蛋白endomucin(EMCN)。从长骨远端横截面上观察,H型血管分布致密,且倾向于在生长板附近发生交联。相反地,L型血管在骨干骨髓腔内形成致密且高度分支的毛细血管网,类似窦状毛细血管网[13-14]。与H型毛细血管相反,L型血管表达低水平的CD31和EMCN。窦状L型毛细血管周围包绕着致密的造血细胞,与中心静脉相连。在这种组织结构下,动脉和远端小动脉不直接将血液运送至窦状L型毛细血管,而是动脉仅与骨骺和骨内膜的H型血管相连。由于骨组织中这种特殊结构的血管系统,血液流经动脉分布至H型毛细血管,再经骨干和骨骺端交界面的窦状L型毛细血管网,最终汇入大的中央静脉。因此,在出生后的小鼠长骨中可检测到不同的代谢环境:骨干由于缺乏动脉的直接供氧和缺乏造血细胞而处于高度缺氧状态,而骨骺相对来说含氧丰富[15]。 此外,骨组织的血管系统含有各类壁细胞。骨髓中,两种管周细胞包绕着窦状L型毛细血管——即瘦素受体阳性细胞(leptin receptor+ cell,LEPR+ cell)和CXCL12丰富的网状细胞(CXCL12-abundant reticular cell,CAR cell)[16-17]。大量证据显示,这些管周细胞在调节造血作用中起着重要作用,它们分泌分子信号如干细胞因子,趋化因子CXCL12和促血管生成素。LEPR+细胞还表达血小板衍生生长受体α(platelet-derived growth factor receptor α,PDGFRα),但不表达血小板衍生生长受体β(platelet-derived growth factor receptor β,PDGFRβ)和神经胶质抗原2(neural/glial antigen2,NG2)[17-18]。这些细胞低表达巢蛋白绿色免疫荧光(nestin-GFP),能分化为不同的间充质细胞系如骨、软骨、脂肪细胞[19]。同软组织一样,骨组织中的动脉被α-平滑肌肌动蛋白阳性(alpha smooth muscle actin-positive,αSMA+ )平滑肌细胞覆盖,并且这些平滑肌细胞也表达NG2[20]。小动脉管周细胞表达NG2和nestin-GFPhigh,也具有能分化为不同的间充质细胞系的潜力[21-22]。骨骺H型毛细血管束也被血小板衍化生长因子Rβ+和NG2+管周细胞包绕,由H型毛细血管的内皮细胞分泌的血小板衍化生长因子B(platelet derived growth factor-B,PDGFB)调控[20]。 2.3 骨组织中的血管形成 骨形成有两种方式即软骨内成骨和膜内成骨。在颅骨和面骨发育过程或骨修复过程中,膜内成骨骨化部位的间充质细胞先分化为成骨细胞,形成骨化点,产生骨组织的纤维和有机基质。钙盐渐次与基质结合分化成骨质,其外围的间充质分化为骨膜。在骨膜下的成骨细胞和破骨细胞的相互作用下,骨即从骨化点逐渐扩展,这一过程称为膜内成骨。这种成骨形式主要发生在扁骨如颅顶骨和面颅诸骨以及部分锁骨。另一种方式,软骨内成骨是由矿化的骨取代软骨,这是一个复杂的过程,由软骨间叶原基增殖的软骨细胞向非增殖的肥大状态分化而启动。在此过程中,间充质细胞分化为软骨细胞。血管入侵和软骨细胞增殖相互协调,进而延长骨。软骨内成骨主要发生在长骨[23-24]。骨组织中的血管系统似乎大部分,甚至可能全部由血管形成生成[25]。 2.3.1 软骨内成血管 脊椎动物骨骼系统的大部分骨以软骨内成骨的方式形成,胚胎期间充质细胞凝聚引发软骨内成骨,生成软骨单元,血管随之入侵软骨,并带来血管周围的骨祖细胞、募集破骨细胞,最终导致软骨单元被吸收并逐渐被骨组织所替代。在鼠科动物的长骨中,在胚胎13.5-14.5 d时血管开始入侵软骨板,血管形成大部分在青春期或发育时期完成。软骨内成血管的过程涉及一系列的事件。首先,未来初级骨化中心(primary ossification center,POC)位点的软骨细胞停止增殖,变肥大,分泌促血管生长因子,刺激血管形成;位于初级骨化中心的骨祖细胞也是促血管生长因子的来源。接着,血管入侵肥大的软骨细胞,形成最初的血管网,并伴随骨化过程。长骨两端的成熟和肥大生长板软骨细胞释放信号,进一步促进血管形成和沿长轴的骨化,最终导致骨单位的扩张延伸。这一过程同时涉及不同的骨骺和骨干毛细血管网的形成。在随后的发育中,血管入侵长骨两端骨骺的软骨细胞,进而启动次级骨化中心(secondary ossification center,SOC)形成[25],见图1。 2.3.2 膜内成血管 通过膜内成骨形成的骨不具有软骨板。但是,和长骨一样,扁骨也是高度血管化的。相较于软骨内成骨和软骨内成血管的研究,膜内成血管却鲜有研究,因而膜内成骨和血管形成的相互作用目前尚不清楚[26-27]。 2.4 影响骨血管形成的因素 提炼简表见表1。 2.4.1 血管内皮生长因子(vascular endothelial growth factor,VEGF) 近30年来,血管内皮生长因子已成为众多研究的焦点。科学家们发现了内皮细胞上的血管内皮生长因子受体——生理和病理性血管生成的关键因子[28-29]。血管内皮生长因子A是调控成血管的主要因子,在肥大软骨细胞中高表达和分泌[30-31]。血管内皮生长因子R2是血管内皮生长因子A的主要受体,二者结合后产"

| [1] Steiner D, Lampert F, Stark GB, et al. Effects of endothelial cells on proliferation and survival of human mesenchymal stem cells and primary osteoblasts. J Orthop Res.2012; 30(10): 1682-1689.[2] Armulik A,Genove G,Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011; 21(2):193-215.[3] Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007; 8(6): 464-478.[4] Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development. 2011; 138(21): 4569-4583.[5] Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12(9): 551-564.[6] Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011; 146(6): 873-887.[7] Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011; 473(7347): 298-307.[8] Kuhnert F, Mancuso MR, Shamloo A, et al. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010; 330(6006): 985-989.[9] Ramasamy SK, Kusumbe AP, Adams RH. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2015;25(3):148-157.[10] Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016; 529(7586): 316-325.[11] Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349-355.[12] Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492): 323-328.[13] Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100(2):174-190.[14] Kopp HG, Avecilla ST, Hooper AT, et al. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology. 2005; 20: 349-356.[15] Ramasamy SK, Kusumbe AP, Wang L, et al. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014; 507(7492): 376-80.[16] Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012; 481(7382): 457-462.[17] Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006; 25(6):977-988.[18] Ugarte F, Forsberg EC. Haematopoietic stem cell niches: new insights inspire new questions. Embo J. 2013; 32(19): 2535-2547.[19] Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154-168.[20] Kusumbe AP, Ramasamy SK, Itkin T, et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016; 532(7599): 380-384.[21] Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013; 502(7473): 637-643.[22] Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010; 466(7308): 829-834.[23] Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100-114.[24] Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 2013; 5(1). a008334.[25] Sivaraj KK, Adams RH. Blood vessel formation and function in bone. Development. 2016; 143(15): 2706-2715.[26] Percival CJ, Richtsmeier JT. Angiogenesis and intramembranous osteogenesis. Dev Dyn. 2013; 242(8): 909-922.[27] Abzhanov A, Rodda SJ, McMahon AP, et al. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007; 134(17): 3133-3144.[28] Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000; 6(4): 389-395.[29] Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003; 9(6): 669-676.[30] Harper J, Klagsbrun M. Cartilage to bone--angiogenesis leads the way. Nature medicine. 1999; 5(6): 617-618.[31] Gerber HP,Vu TH,Ryan AM,et al.VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature Med.1999; 5(6): 623-628.[32] Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006; 7(5): 359-371.[33] Geiger F, Lorenz H, Xu W, et al. VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Bone. 2007; 41(4): 516-522.[34] Keramaris NC, Calori GM, Nikolaou VS, et al. Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury. 2008; 39 Suppl 2: S45-57.[35] Kasten P,Beverungen M,Lorenz H,et al.Comparison of platelet-rich plasma and VEGF-transfected mesenchymal stem cells on vascularization and bone formation in a critical-size bone defect. Cells Tissues Organs. 2012;196(6): 523-533.[36] Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010; 10(2): 116-129.[37] Hankenson KD, Dishowitz M, Gray C, et al. Angiogenesis in bone regeneration. Injury. 2011; 42(6): 556-561.[38] Kozhemyakina E,Lassar AB,Zelzer E.A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015; 142(5): 817-831.[39] Ornitz DM, Marie PJ. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015; 29(14): 1463-1486.[40] Itkin T, Gur-Cohen S, Spencer JA, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016; 532(7599): 323-328.[41] Camelo M, Nevins ML, Schenk RK, et al. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent. 2003; 23(3): 213-225.[42] Nevins M,Camelo M,Nevins ML,et al.Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003; 74(9): 1282-1292.[43] Guo P, Hu B, Gu W, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol.2003; 162(4): 1083-1093.[44] Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014; 15(12): 786-801.[45] Lu P, Takai K, Weaver VM, et al. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011; 3(12). pii: a005058.[46] Chen TT, Luque A, Lee S, et al. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol. 2010; 188(4): 595-609.[47] Stickens D, Behonick DJ, Ortega N, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131(23): 5883-5895.[48] Bentovim L,Amarilio R, Zelzer E. HIF1alpha is a central regulator of collagen hydroxylation and secretion under hypoxia during bone development. Development. 2012; 139(23): 4473-4483.[49] Dunwoodie SL.The role of hypoxia in development of the Mammalian embryo. Developmental Cell. 2009; 17(6): 755-773.[50] Schipani E, Ryan HE, Didrickson S, et al. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001; 15(21): 2865-2876.[51] Maes C,Clemens TL.Angiogenic-osteogenic coupling: the endothelial perspective. Bonekey Rep. 2014; 3: 578.[52] Riddle RC,Khatri R,Schipani E,et al.Role of hypoxia-inducible factor-1alpha in angiogenic-osteogenic coupling. J Mol Med (Berl). 2009; 87(6): 583-590.[53] Schipani E, Maes C, Carmeliet G, et al. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res. 2009; 24(8):1347-1353.[54] Engin F, Lee B. NOTCHing the bone: insights into multi-functionality. Bone. 2010; 46(2): 274-280.[55] Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007; 21(20): 2511-2524.[56] Jakobsson L, Bentley K, Gerhardt H. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochem Soc Trans. 2009; 37(Pt 6): 1233-1236.[57] Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nature Med. 2001; 7(6): 706-711.[58] Dohle E, Fuchs S, Kolbe M, et al. Comparative study assessing effects of sonic hedgehog and VEGF in a human co-culture model for bone vascularisation strategies. Eur Cell Mater. 2011; 21: 144-156.[59] Ho JE, Chung EH, Wall S, et al. Immobilized sonic hedgehog N-terminal signaling domain enhances differentiation of bone marrow-derived mesenchymal stem cells. J Biomed Mater Res A. 2007; 83(4):1200-1208.[60] Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011; 10(12): 945-963.[61] Ivkovic S, Yoon BS, Popoff SN, et al. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003; 130(12): 2779-2791.[62] Liu ES, Raimann A, Chae BT, et al. c-Raf promotes angiogenesis during normal growth plate maturation. Development. 2016; 143(2): 348-355.[63] Hiraki Y, Shukunami C. Angiogenesis inhibitors localized in hypovascular mesenchymal tissues: chondromodulin-I and tenomodulin. Connect Tissue Res. 2005; 46(1): 3-11.[64] Ikegami D, Akiyama H, Suzuki A, et al. Sox9 sustains chondrocyte survival and hypertrophy in part through Pik3ca-Akt pathways. Development. 2011; 138(8): 1507-1519.[65] Eshkar-Oren I, Viukov SV, Salameh S, et al. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development. 2009; 136(8): 1263-1272. |
| [1] | Zhang Lichuang, Xu Hao, Ma Yinghui, Xiong Mengting, Han Haihui, Bao Jiamin, Zhai Weitao, Liang Qianqian. Mechanism and prospects of regulating lymphatic reflux function in the treatment of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1459-1466. |
| [2] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [3] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [4] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [5] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [6] | Zhang Yujie, Yang Jiandong, Cai Jun, Zhu Shoulei, Tian Yuan. Mechanism by which allicin inhibits proliferation and promotes apoptosis of rat vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1080-1084. |
| [7] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [8] | Mo Weibin, Huang Tianchang, Zeng Zhiwei, Yan Linbo. Effects of Pueraria lobata flavonoids on expressions of beta-catenin and glycogen synthase kinase 3beta in the brain of rats undergoing exhaustive exercise after long endurance exercise [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 736-741. |
| [9] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [10] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [11] | Baibujiafu·Yelisi, Renaguli·Maihemuti, Aizimaitijiang·Saiyiti, Wang Junxiang, Nijiati·Tuerxun. Stress analysis of maxillary central incisor crown implant restoration in different occlusal modes [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 567-572. |
| [12] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [13] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [14] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [15] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||