Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (21): 3432-3437.doi: 10.3969/j.issn.2095-4344.2017.21.025
Previous Articles Next Articles
Stem cell transplantation for inflammatory bowel diseases
Li Qing-qing1, 2, Liu Jian-kun1, Pan Xing-hua1
- 1Cell Biological Therapy Center of Kunming General Hospital of Chengdu Military Area of Chinese PLA, Cell Biological Medicine Integrated Engineering Laboratory of State and Regions in Yunnan Province, Stem Cell Engineering Laboratory of Yunnan Province, Key Laboratory of Stem Cells and Regenerative Medicine of Kunming City, Kunming 650032, Yunnan Province, China; 2Kunming General Hospital of Chengdu Military Region, Kunming Medical University Clinical College, Kunming 650031, Yunnan Province, China
-
Revised:2017-02-10Online:2017-07-28Published:2017-08-02 -
Contact:Pan Xing-hua, M.D., Chief physician, Master’s supervisor, Cell Biological Therapy Center of Kunming General Hospital of Chengdu Military Area of Chinese PLA, Cell Biological Medicine Integrated Engineering Laboratory of State and Regions in Yunnan Province, Stem Cell Engineering Laboratory of Yunnan Province, Key Laboratory of Stem Cells and Regenerative Medicine of Kunming City, Kunming 650032, Yunnan Province, China -
About author:Li Qing-qing, Studying for master’s degree, Cell Biological Therapy Center of Kunming General Hospital of Chengdu Military Area of Chinese PLA, Cell Biological Medicine Integrated Engineering Laboratory of State and Regions in Yunnan Province, Stem Cell Engineering Laboratory of Yunnan Province, Key Laboratory of Stem Cells and Regenerative Medicine of Kunming City, Kunming 650032, Yunnan Province, China -
Supported by:the National Science and Technology Support Program of China, No. 2014BIO1B01
CLC Number:
Cite this article
Li Qing-qing, Liu Jian-kun, Pan Xing-hua. Stem cell transplantation for inflammatory bowel diseases[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(21): 3432-3437.
share this article
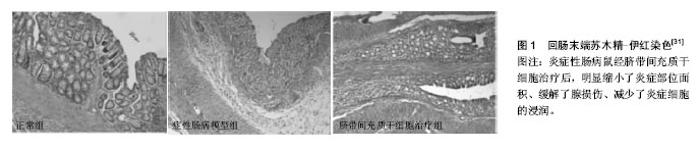
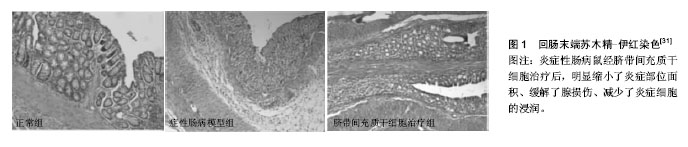
2.1 胚胎干细胞 胚胎干细胞从分化潜能上看属全能干细胞,由于其涉及伦理道德方面的问题,因此,相对其他类型的干细胞来讲,将胚胎干细胞用于治疗炎症性肠病的研究报道并不多见。Srivastava等[9]发现用胚胎干细胞可以减轻IL10-/- KO鼠肠炎模型的炎症。白细胞介素12由激活的抗原提呈细胞所产生,它可促进T细胞的分化、增殖与炎症因子的产生。经胚胎干细胞移植后的IBD动物模型,其白细胞介素12血清水平正常化,成簇的胚胎干细胞聚集在炎症部位,而脾脏、血液、骨髓等部位未检测到胚胎干细胞。 2.2 成体干细胞 2.2.1 造血干细胞 造血干细胞是最早用于治疗IBD的干细胞[10],它能在肠黏膜受损处定植且可分化为肠上皮,同时发挥免疫平衡的作用[11]。Cassinotti等[12]将自身造血干细胞注射到患者体内,随访观察大约16.5个月,3/4的患者病情得到了有效控制,在该期间内,这些患者没有进行任何其他的治疗。Hommes等[13]注意到2例接受了自身造血干细胞移植的克罗恩病患者在随访期间内实现了临床症状的缓解,而未经该治疗的1例患者出现了活动性的克罗恩病。Snowden等[14]回顾了相关的记录,发现造血干细胞移植可致患者死亡,感染是主要原因之一。有人尝试用自身造血干细胞移植治疗患有顽固性克罗恩病而又不愿接受手术治疗的患者,结果发现移植组与标准治疗组(空白对照)在维持疾病缓解方面并无显著差别,移植组产生的不良反应事件几乎是空白对照组的2倍,移植组中1例患者死亡,这些研究并不支持造血干细胞在难治性克罗恩病人群中的广泛应用,毕竟造血干细胞存在显著的毒性[15]。脐血干细胞是一类特殊的造血干细胞[16]。脐血干细胞通过Nucleotide-binding oligomerization domain- containing 2 (NOD2)信号通路的激活产生PGE2抑制单核细胞的增殖,从而有效治疗肠炎模型鼠[17]。NOD2是一种位于细胞内的受体,它在固有免疫中发挥着十分重要的作用[18]。彭凯玥等[19]用脐血干细胞移植治疗白细胞介素10受体A基因突变导致的炎症性肠病,通过Sanger测序及蛋白功能检测验证基因突变位点已修复,白细胞介素10信号通路正常化,患儿的大便慢慢成型,体质量也逐渐增加,这项研究揭示干细胞可以修复患者的基因。 2.2.2 间充质干细胞 间充质干细胞不同于造血干细胞,它们的表面分子一般为CD13,CD29,CD44,CD73,CD90,CD166,CXCL12,Toll样受体。间充质干细胞可来源于骨髓、大脑、脂肪组织、外周血、眼角膜、胸腺、脾脏、输卵管、胎盘、脐带及脐血等。间充质干细胞的主要特性为免疫调节和归巢到缺血或损伤部位[20]。有研究认为,间充质干细胞治疗优于造血干细胞治疗[21]。外周血干细胞移植对少部分克罗恩病患者有效,且移植前需对患者骨髓进行处理,而间充质干细胞可以逃避免疫识别,从而无需行清髓处理[22]。关于间充质干细胞应采用的移植方式,争议较多,有学者认为腹腔注射比静脉注射的疗效更好[23],也有学者认为二者差别不大[24]。近几年,大量的临床试验证明了间充质干细胞的安全性[25]。Taddio等[26]表示用ITN-γ预处理过的间充质干细胞治疗克罗恩病患者,并没什么作用,但此前有人报道IFN-γ可活化间充质干细胞,从而增强间充质干细胞在体内或体外的免疫抑制作用[27]。 脐带间充质干细胞:脐带间充质干细胞所处的环境相对来说比较原始,因此这种细胞拥有较强的增殖与分化潜能,另外,较低的免疫原性与取材的简易性等,使之倍受科研工作者青睐。有学者证实脐带间充质干细胞通过下调细胞因子、其他的炎症递质以及上调细胞间紧密连接蛋白来缓解葡聚糖硫酸钠诱导的溃疡性结肠炎病情[28]。用人脐带间充质干细胞经尾静脉注射至氧化偶氮甲烷(AOM)和葡聚糖硫酸钠(DSS)诱导的结肠炎相关的结直肠癌动物模型体内,结果显示,脐带间充质干细胞迁移至结肠组织而且经Smad2信号通路能诱导幼稚T细胞向调节性T细胞分化,最终抑制了结肠炎与结直肠癌的发展与恶化[29]。Banerjee等[30]发现脐带间充质干细胞在葡聚糖硫酸钠诱导的免疫缺陷急性肠炎模型鼠中发挥了直接的防御性功能(图1),而无需借助T细胞的免疫调节特性,另外实验结果也提示金属蛋白酶与内质网应激可能成为IBD的治疗靶标。有研究暗示越老的间充质干细胞即传代次数越多的细胞,其形态学变化、基因表达谱更加丰富,而且免疫抑制特性增强,这为治疗性间充质干细胞的选择提供了理论依据[31]。Fuenzalida等[32]证实脐带间充质干细胞容易受到极化条件的影响,在葡聚糖硫酸钠诱导的结肠炎动物模型中,他们发现经聚肌苷酸胞苷酸即poly(I:C)预处理的脐带间充质干细胞较纯粹的脐带间充质干细胞或经脂多糖预处理的脐带间充质干细胞而言,能显著改善动物的临床症状及其肠壁的组织病理表现,预示着聚肌苷酸胞苷酸预处理的脐带间充质干细胞可能成为治疗IBD的可行细胞药物。当通过导入相关基因使人脐带间充质干细胞过表达白细胞介素17受体样分子(IL-17RLM)时,发现在一定浓度范围内,该类细胞对三硝基苯磺酸(TNBS)诱导的结肠炎小鼠模型的脾脏淋巴细胞Th1、Th17的抑制效果较普通人脐带间充质干细胞更明显,这为优化细胞打下了一定的基础[33]。众所周知,IFN-γ能增强间充质干细胞的免疫抑制特性。Chen等[34]将能表达IFN-γ的质粒转染间充质干细胞,以达到基因修饰的目的,其较常规的间充质干细胞能明显抑制T细胞的增殖,更有趣地是,将IFN-γ- MSCs注射至肠炎模型动物体内,发现此类细胞更能有效地缓解病情;另外,IFN-γ-MSCs增加了Foxp3+Tregs和Th2细胞的数量,锐减了结肠中的炎症细胞因子,1例顽固性结肠炎患者静脉输注脐带来源间充质干细胞后,患者腹痛及顽固性腹泻消失且无任何不良反应记录,至于远期疗效如何,研究人员没有给出相关的随访记录[35]。 骨髓间充质干细胞:人骨髓间充质干细胞调节T细胞的功能已为大家所知,但其分子机制并没有完全阐释清楚。近来,有人将T细胞与骨髓间充质干细胞共培养后发现骨髓间充质干细胞能显著降低T细胞活化标记物CD38以及共刺激分子CD134、CD154的表达,但CD27的表达不会受到影响,并且骨髓间充质干细胞可以抑制T细胞增殖,说明骨髓间充质干细胞可以使T细胞应答处于免疫耐受状态[36]。国内研究人员用胎儿肠壁结缔组织成功诱导骨髓间充质干细胞,实现了骨髓间充质干细胞向肠上皮细胞的分化[37]。另有报道,间充质干细胞能向结肠血管内皮细胞分化从而有助于血管生成,同时有效抑制炎症反应[38]。运用慢病毒技术使骨髓间充质干细胞表达绿色荧光蛋白(Ad-GFP-BMSCs)或共表达CXCR4和GFP(Ad-CXCR4-BMSCs)[39],实验结果显示注射过Ad-CXCR4-BMSCs的结肠炎模型鼠病变部位的IFN-γ、TNF-α、IL-6 mRNA表达显著下降,而IL-10 mRNA表达显著上升,STAT-3和磷酸化STAT-3蛋白表达显著下降,刘星星等[39]推测过表达CXCR4的骨髓间充质干细胞通过免疫调节与抗炎等机制来明显改善实验性结肠炎症状,而注射Ad-GFP-BMSCs的实验模型大鼠其疗效不如前者。有人在一项Ⅱ期临床试验中运用异基因骨髓来源间充质干细胞治疗顽固性克罗恩病,15例患者中8例患者有临床症状缓解,7例患者其内镜下出现组织病理学改善,从而证明此类细胞具有一定的临床疗效[22]。 脂肪来源的干细胞:有研究显示,来自人脐血的血小板溶解产物能增强脂肪间充质干细胞的治疗效果[40]。将脂肪来源干细胞植入6例术后肠皮肤瘘患者的发病部 位[41],12周左右瘘管闭全率达83.3%,24周时达100%,在此临床试验中,研究人员从臀部脂肪组织中获取了大量的脂肪间充质干细胞而不需要将细胞进行培养或多次传代。瘘管是克罗恩病患者常见的一种典型并发症,常规的治疗对其难以奏效且容易复发。在Ⅰ期临床试验中,研究人员证实了脂肪间充质干细胞治疗并发瘘管的克罗恩病安全有效,Ⅱ期临床研究中发现自身脂肪间充质干细胞治疗该类患者的有效率达80%以上,在随访的1年内,大多数人瘘管闭合且没有复发,也没有其他的不良反应发生[42]。但一项大规模的Ⅲ期研究发现用自身来源脂肪间充质干细胞治疗肛门瘘时,并没有效果[43]。 2.2.3 肠道干细胞 肠道干细胞属成体干细胞,一般分布于肠道隐窝。肠道上皮细胞的更新过程高度依赖肠道干细胞的分裂与补充[44]。Lgr5蛋白被认为是肠道干细胞的标记物[45]。有人认为肠道干细胞是治疗IBD的最佳细胞。因为提取与培养困难,目前肠道干细胞用于治疗IBD的研究报道较少。Shaker等[46]将来自结肠的干细胞在体外培养成由上皮组织构成的微小器官,然后将其植入到大鼠肠道损伤处,发现该器官类似物黏附到病变处而且产生了与正常结肠上皮高度一致的上皮组织,此移植物支持上皮长期再生。 2.2.4 体细胞重编程 体细胞重编程是指已分化的体细胞在一定条件下可逆转恢复到全能或多能的状态,形成胚胎干细胞系或进一步发育成一个新个体的过程。在2006年,体细胞重编程这一概念,首先由Takahashi与Yamanaka 提出,他们发现4种转录因子,即Oct4,Sox2,Klf4以及c-Myc能使大鼠体细胞逆转为诱导多能干细胞[47]。有一些人设想将肠道干细胞改造成多能干细胞。这个过程需要借助侵袭性细菌载体来完成:把携带有能编码多能性因子的重编程基因的细菌转移至损伤组织。这种细菌可以在肠道表面定植,它可以把基因转染到宿主的目标器官或组织中,从而实现细胞重编程。在体内环境下,因具备体外培养所不能提供的必要分子以及空间因素,即微环境,将有利于这种“人工多能干细胞”的生长发育与分化[48]。目前为止,未见有人将体细胞重编程技术用于IBD的研究。"
| [1] Jansson A, Pernestig AK, Nilsson P, et al. Toward quantifying the thymic dysfunctional state in mouse models of inflammatory bowel disease .Inflamm Bowel Dis. 2013;19(4):881-888.[2] Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860-867. [3] 王玉芳,欧阳钦,胡仁伟,等.炎症性肠病流行病学研究进展[J].胃肠病学,2013,18(1): 48-51.[4] Wallace KL, Zheng LB, Kanazawa Y, et al. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014; 20(1):6-21.[5] Pedros C, Gaud G, Bernard I, et al. An Epistatic Interaction between Themis1 and Vav1 Modulates Regulatory T Cell Function and Inflammatory Bowel Disease Development. J Immunol. 2015;195(4):1608-1616. [6] 李家玉,卢启明.炎症性肠病的内科治疗进展[J].甘肃医药,2015, 34(2):98-101. [7] 黄缘.炎症性肠病的治疗现状[J].世界华人消化杂志,2015, 23(26):4150-4154.[8] Larijani B, Esfahani EN, Amini P, et al. Stem cell therapy in treatment of different diseases. Acta Med Iran. 2012;50(2): 79-96.[9] Srivastava AS, Feng Z, Mishra R, et al. Embryonic stem cells ameliorate piroxicam-induced colitis in IL10-/- KO mice. Biochem Biophys Res Commun. 2007;361(4):953-959.[10] 徐隽,智发朝.炎症性肠病治疗新进展—干细胞治疗[J]. 现代消化及介入诊疗,2015,20(4):447-451. [11] Gazouli M, Roubelakis MG, Theodoropoulos GE. Stem cells as potential targeted therapy for inflammatory bowel disease. Inflamm Bowel Dis. 2014;20(5):952-955.[12] Cassinotti A, Annaloro C, Ardizzone S, et al. Autologous haematopoietic stem cell transplantation without CD34+ cell selection in refractory Crohn's disease. Gut. 2008;57(2): 211-217.[13] Hommes DW, Duijvestein M, Zelinkova Z, et al. Long-term follow-up of autologous hematopoietic stem cell transplantation for severe refractory Crohn's disease. J Crohns Colitis. 2011;5(6):543-549.[14] Snowden JA, Saccardi R, Allez M, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47(6):770-790.[15] Hawkey CJ, Allez M, Clark MM, et al. Autologous Hematopoetic Stem Cell Transplantation for Refractory Crohn Disease: A Randomized Clinical Trial. JAMA. 2015;314(23): 2524-2534.[16] 李楠.脐带间充质干细胞与炎症性肠病[J].山东医药,2011, 51(16):8-9.[17] Kim HS, Shin TH, Lee BC, et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology. 2013;145(6): 1392-1403.[18] Corridoni D, Kodani T, Rodriguez-Palacios A, et al. Dysregulated NOD2 predisposes SAMP1/YitFc mice to chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2013;110(42):16999-17004.[19] 彭凯玥,钱晓文,吴冰冰.脐血干细胞移植治疗白介素10受体A基因突变导致的极早发型炎症性肠病1例病例报告并文献复习[J].中国循证儿科杂志,2016,11(3):171-176.[20] Rahimzadeh A, Mirakabad FS, Movassaghpour A, et al. Biotechnological and biomedical applications of mesenchymal stem cells as a therapeutic system. Artif Cells Nanomed Biotechnol. 2016;44(2):559-570.[21] Irhimeh MR, Cooney J. Management of Inflammatory Bowel Disease Using Stem Cell Therapy. Curr Stem Cell Res Ther. 2016;11(1):72-77.[22] Forbes GM, Sturm MJ, Leong RW, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12(1):64-71.[23] Castelo-Branco MT, Soares ID, Lopes DV, et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7(3):e33360.[24] 葛翠翠,王慧娜,杜丽欣,等.人脐带间充质干细胞对炎症性肠病小鼠模型的治疗作用[J].生物技术通讯,2014,25(6):813-816.[25] Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012; 7(10):e47559.[26] Taddio A, Tommasini A, Valencic E, et al. Failure of interferon-γ pre-treated mesenchymal stem cell treatment in a patient with Crohn's disease. World J Gastroenterol. 2015;21(14):4379- 4384.[27] Valencic E, Piscianz E, Andolina M, et al. The immunosuppressive effect of Wharton's jelly stromal cells depends on the timing of their licensing and on lymphocyte activation. Cytotherapy. 2010;12(2):154-160.[28] Lin Y, Lin L, Wang Q, et al. Transplantation of human umbilical mesenchymal stem cells attenuates dextran sulfate sodium-induced colitis in mice. Clin Exp Pharmacol Physiol. 2015;42(1):76-86.[29] Tang RJ, Shen SN, Zhao XY, et al. Mesenchymal stem cells-regulated Treg cells suppress colitis-associated colorectal cancer. Stem Cell Res Ther. 2015;6:71.[30] Banerjee A, Bizzaro D, Burra P, et al. Umbilical cord mesenchymal stem cells modulate dextran sulfate sodium induced acute colitis in immunodeficient mice. Stem Cell Res Ther. 2015;6:79.[31] Zhuang Y, Li D, Fu J, et al. Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: immunomodulatory ability is enhanced in aged cells. Mol Med Rep. 2015;11(1):166-174.[32] Fuenzalida P, Kurte M, Fernández-O'ryan C, et al. Toll-like receptor 3 pre-conditioning increases the therapeutic efficacy of umbilical cord mesenchymal stromal cells in a dextran sulfate sodium-induced colitis model. Cytotherapy. 2016; 18(5):630-641.[33] 郭婧,晁康,康健,等.过表达IL-17RLM的人脐带间充质干细胞对TNBS诱导的结肠炎小鼠脾脏淋巴细胞的免疫调节作用[J].中国病理生理杂志,2016,32(6):961-970.[34] Chen Y, Song Y, Miao H, et al. Gene delivery with IFN-γ-expression plasmids enhances the therapeutic effects of MSCs on DSS-induced mouse colitis. Inflamm Res. 2015; 64(9):671-681.[35] 刘静,韩冬梅,薛梅,等.脐带间充质干细胞输注治疗顽固性溃疡性结肠炎[J].组织工程与重建外科杂志,2011,7(5):258-260.[36] Fayyad-Kazan H, Faour WH, Badran B, et al. The immunomodulatory properties of human bone marrow-derived mesenchymal stromal cells are defined according to multiple immunobiological criteria. Inflamm Res. 2016;65(6):501-510.[37] 段江洁,王蓓,张建华,等.胎儿肠壁结缔组织诱导骨髓间充质干细胞向肠上皮细胞的分化[J]. 第三军医大学学报,2008,30(18): 1718-1721.[38] 张夏梦,寿折星,石月萍,等.骨髓间充质干细胞对溃疡性结肠炎大鼠结肠组织血管内皮的修复作用[J].世界华人消化杂志,2013, 21(28):2908-2914.[39] 刘星星,范恒,唐庆,等.过表达CXCR4的间充质干细胞缓解实验性结肠炎[J]. 世界华人消化杂志,2016, 24(8):1233-1240.[40] Forte D, Ciciarello M, Valerii MC, et al. Human cord blood-derived platelet lysate enhances the therapeutic activity of adipose-derived mesenchymal stromal cells isolated from Crohn's disease patients in a mouse model of colitis. Stem Cell Res Ther. 2015;6:170.[41] Mizushima T, Takahashi H, Takeyama H, et al. A clinical trial of autologous adipose-derived regenerative cell transplantation for a postoperative enterocutaneous fistula. Surg Today. 2016;46(7):835-842.[42] Lee WY, Park KJ, Cho YB, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn's fistula. Stem Cells. 2013;31(11):2575-2581.[43] Herreros MD, Garcia-Arranz M, Guadalajara H, et al. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long-term evaluation. Dis Colon Rectum. 2012;55(7):762-772.[44] 祝芳,曲波,金世柱,等.肠道干细胞与炎症性肠病的关系[J].胃肠病学和肝病学杂志,2013,22(8):717-719.[45] Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003-1007.[46] Shaker A, Rubin DC. One step closer to gut repair. Nature. 2014;485(7397):181-182.[47] Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676.[48] Wagnerova A, Gardlik R. In vivo reprogramming in inflammatory bowel disease. Gene Ther. 2013;20(12): 1111-1118.[49] Munir H, McGettrick HM. Mesenchymal Stem Cell Therapy for Autoimmune Disease: Risks and Rewards. Stem Cells Dev. 2015;24(18):2091-2100. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [5] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [6] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [7] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [8] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [9] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [10] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [11] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [12] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| [13] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [14] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [15] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||