Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (12): 1926-1932.doi: 10.3969/j.issn.2095-4344.2017.12.021
Previous Articles Next Articles
Application and progress of co-culture systems in cartilage tissue engineering
Zhang Yu1, Liu Shu-yun1, Guo Wei-min1, Hao Chun-xiang2, Wang Ming-jie1, Lu Liang3, Lu Shi-bi1, Guo Quan-yi1
- 1Institute of Orthopaedics, Chinese PLA General Hospital, the Beijing Key Laboratory of Regenerative Medicine in Orthopaedics, the PLA Key Laboratory of Musculoskeletal Trauma & War Injuries, Beijing 100853, China; 2Department of Anesthesiology, the General Hospital Of Chinese PLA, Beijing 100853, China; 3Second Department of Orthopaedics, Anhui Provincial Hospital, Hefei 230041, Anhui Province, China
-
Received:2016-12-01Online:2017-04-28Published:2017-05-16 -
Contact:Guo Quan-yi, Chief physician, Associate professor, Master’s supervisor, Institute of Orthopaedics, Chinese PLA General Hospital, the Beijing Key Laboratory of Regenerative Medicine in Orthopaedics, the PLA Key Laboratory of Musculoskeletal Trauma & War Injuries, Beijing 100853, China -
About author:Zhang Yu, Studying for master’s degree, Institute of Orthopaedics, Chinese PLA General Hospital, the Beijing Key Laboratory of Regenerative Medicine in Orthopaedics, the PLA Key Laboratory of Musculoskeletal Trauma & War Injuries, Beijing 100853, China -
Supported by:the National Natural Science Foundation of China, No. 81472092; the National High-Tech Research & Development Program of China (863 Program), No. 2015AA020303
CLC Number:
Cite this article
Zhang Yu1, Liu Shu-yun1, Guo Wei-min1, Hao Chun-xiang2, Wang Ming-jie1, Lu Liang3, Lu Shi-bi1, Guo Quan-yi1. Application and progress of co-culture systems in cartilage tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(12): 1926-1932.
share this article
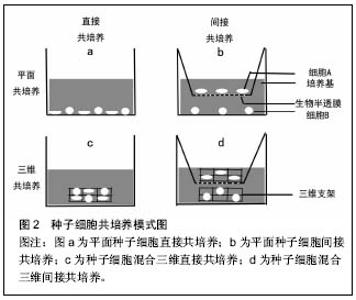
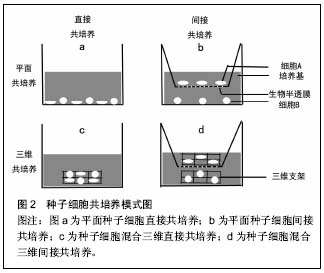
2.1 共培养概念的提出及在软骨组织工程中的研究 近些年来,已经有报道细胞间的信号在细胞的生物学行为中发挥着重要的作用[6]。在体内,细胞的新陈代谢和功能的发挥时刻受到来自微环境的信息调控,包括来自周围细胞、细胞外基质或因子中的生化信息、生物力学和生物电刺激信号等[7-8]。当细胞脱离机体内环境,通过分离、消化及纯化在体外环境单种培养时,导致细胞失去了天然细胞微环境的作用,其相应的天然生物学特性也因此而丢失。在共培养系统中,能够为种子细胞提供所需的物理条件、化学营养物质的同时,通过加入一种或以上的种子细胞来提供相应的生物刺激信息,模拟体内生物微环境,以维持细胞的正常代谢和功能[9]。在许多领域研究证实细胞间相互作用影响着细胞结构、细胞极性、细胞增殖和生化功能[10-11]。软骨组织工程中,相对于单种细胞培养,共培养系统通过构建的仿生体内微环境表现出其独特的优势。一方面,陪伴细胞(如间充质干细胞)通过分泌营养因子或细胞间的信息交流促进软骨细胞增殖和延迟去分化[12]。同时,软骨细胞能够诱导具有多项分化潜能的间充质干细胞分化成软骨[13]。另一方面,共培养降低了软骨组织工程中软骨细胞的需求量,减少了体外扩增培养时间,尽可能的降低了软骨细胞原始表型的丢失。然而,共培养系统是复杂的,其作用机制尚不是非常清楚,存在争议。因此,更好的理解共培养作用机制对于优化软骨组织工程共培养系统,增强组织工程软骨生物学特性是十分必要的。 共培养技术的基本方法是应用两种及以上的种子细胞或材料的共同培养,通过不同种细胞分泌不同细胞因子、细胞间信息交流及细胞与细胞外基质的相互作用,最终目的是达到一个天然的协同作用使他们之间形成优势互补,劣势消除的理想效果。经过近40年的发展,在软骨组织工程领域的共培养已经取得了令人满意的成就。但是,不同的共培养系统常常会得到不同甚至相反的结论。有学者报道认为在软骨细胞与间充质干细胞共培养,可以通过营养作用刺激软骨细胞增殖促进新生软骨形成,而间充质干细胞本身并没有诱导分化成软骨[14]。另外,间充质干细胞能够通过分泌细胞因子和生长因子发挥抗炎和免疫调节作用,提供合适的微环境提高软骨细胞的细胞活性。然而,Acharya等[15]的研究结果中发现,间充质干细胞促进软骨细胞增殖的同时,发现其也发生了成软骨分化,表现为软骨相关基因表达水平上调和蛋白的高合成。导致研究结果层次不齐,甚至相矛盾的原因可能是多方面的,包括共培养系统中种子细胞的来源不同,如表型最原始的原代软骨细胞、已经发生去分化的软骨细胞或不同组织来源的间充质干细胞等。另外,也可能由于共培养条件的差异所致,如三维立体共培养或平面共培养、直接混合共培养或间接共培养、种子细胞比率及生物材料的不同等均是影响实验结果的影响因素之一。 2.2 软骨组织工程共培养系统中各成分的分析 2.2.1 种子细胞的来源 种子细胞是组织工程中最为重要的组成成分,其生物学状态的好与坏对组织工程的结果起到决定性作用。在软骨组织工程中,种子细胞的来源主要包括实质细胞和间充质干细胞。实质细胞主要包括原代软骨细胞、传代后的软骨细胞、骨关节炎软骨细胞和软骨下骨细胞。间充质干细胞主要来源于骨髓血、滑膜组织、脂肪组织、脐带及脐带血等组织。 间充质干细胞:间充质干细胞被广泛应用到软骨组织工程共培养系统中与软骨细胞共培养,主要体现以下优 势[1,16]:①来源广泛且为永生细胞;②具有自我更新和多系分化潜能;③免疫调节功能和低的免疫原性等。这些特性在体内组织的再生与修复中扮演着重要的角色。 不同组织来源的间充质干细胞在共培养系统的应用即有其独特的优势,也面临着一些挑战。在共培养系统中,骨髓间充质干细胞与软骨细胞共培养,骨髓间充质干细胞通过营养作用促进软骨细胞的增殖,延迟软骨细胞的去分化[17-18]。Ouyang等[19]发现和软骨细胞共培养的骨髓单个核细胞也能够延迟软骨细胞的去分化。相对于骨髓干细胞,脂肪间充质干细胞有类似的分化成软骨潜能和克隆增殖能力[15]。另外,脂肪间充质干细胞可以一次获得大量的细胞数,而不需经过体外扩增的优势,有利于临床转化和应用。相比较其他组织来源的间充质干细胞,据报道滑膜间充质干细胞具有更优越的增殖活性及分化成软骨潜能。Zhang等[20]应用滑膜间充质干细胞与经过转化生长因子β3转染的软骨细胞在藻酸盐水凝胶中共培养,发现不仅诱导滑膜干细胞分化成软骨,而且很好的维持了软骨细胞的天然表型。但是,诱导成软骨的滑膜间充质干细胞仍然保持着自身的成纤维细胞特性,这需要未来的研究进一步评估其在共培养系统中的应用价值。胚胎间充质干细胞是最原始的干细胞,可被诱导分化为机体几乎所有的细胞类型。人胚胎干细胞拥有较高的端粒酶活性,具有很强的长期增殖潜能和定向分化能力。Vats等[21]首次报道人胚胎干细胞与软骨细胞共培养后被诱导分化成软骨细胞。与单纯人胚胎干细胞的培养组相比,共培养系统中的人胚胎干细胞能够分泌更多的细胞外基质,如糖胺多糖及Ⅱ型胶原等。由于来源于骨髓、滑膜及脂肪组织的间充质干细胞会给患者带来二次损伤,且细胞的活性受到患者自身体质的影响,而来源于胚胎组织的胚胎干细胞数量受限又带来伦理争议等,这些也一定程度的影响了它们的推广和应用。来源于围产期组织的间充质干细胞,在一定程度上解决了以上问题而成为当前研究的热点。Pereira等[22]用人脐带间充质干细胞与人关节软骨细胞以微球方式共培养,发现人脐带间充质干细胞能够诱导分化成软骨,获得较满意的组织工程软骨。另外,围产期组织来源的广泛,对人体不会造成伤害,又不受伦理约束,其被认为是介于胚胎干与成体间充质干细胞之间的年轻有活力的种子细胞[23-24],因此其有望成为未来该领域具有应用前景的种子细胞之一。 实质细胞:共培养技术已经被尝试用于解决单纯的软骨损伤和骨软骨复合伤的问题中,如骨关节炎和软骨-软骨下骨复合损伤等。在骨关节炎中,软骨细胞一定程度上失去了原有的表型而发生了去分化。相对于原代软骨细胞,发生去分化的软骨细胞其细胞表面标志物已经发生了很大的改变,包括整合蛋白、合成代谢调节因子等[25-28]。原代软骨细胞与去分化的软骨细胞共培养,可以逆转去分化的软骨细胞反分化[29]。这说明,临床中可以保留损伤或退变的软骨组织或细胞与原代软骨细胞共培养,用来修复损伤软骨组织。这不仅充分利用了损伤的软骨组织,而且也很大程度上减少了对正常软骨组织的需求。 由于共培养系统具有多种细胞共同在同一环境培养的独特优势,而骨软骨复合伤以多种组织多种细胞的同时受损为特点,所以近年来,共培养技术应用于骨软骨复合伤修复的探索也成为研究的热点之一[30-33],希望能为骨软骨复合伤带来有效的修复效果。Jiang等[34]研究称,在软骨细胞与成骨细胞的共培养系统中发现,相对于单纯软骨细胞培养组,共培养组的软骨细胞外基质糖胺聚糖明显合成降低。另外,在单纯成骨细胞培养组中,成骨细胞的钙化程度更高。这一研究结果也许可以为骨软骨过渡区的再生提供有效解决途径。 2.2.2 共培养细胞混合比率 在共培养系统中软骨细胞与间充质干细胞的混合比率,可能会通过影响细胞因子的分泌量而影响共培养细胞的细胞行为,如间充质干细胞的成软骨分化[5]。随着软骨细胞比率的增加,Ⅱ型胶原mRNA 的表达水平也显著提高[23]。相反,提高间充质干细胞的比率会导致Ⅰ型和Ⅹ型胶原mRNA的表达水平上调[18,35]。因此,选择最佳的种子细胞混合共培养比率,使共培养结果向着需要的方向发展显得更为重要。Mo等[36]报道,间充质干细胞与软骨细胞共培养的混合比率通过细胞间相互作用影响着细胞的分化和功能。随着间充质干细胞与软骨细胞共培养时间的延长,软骨细胞样外基质的合成增加,同时Ⅱ型胶原基因的表达水平上调。Qing等[35]报道了用兔软骨细胞与骨髓干细胞以2∶1的比率混合共培养为最佳比率,认为软骨细胞分泌的转化生长因子β、骨形态发生蛋白及胰岛样生长因子1在促进骨髓干细胞成软骨方面起到重要作用。Sabatino等[28]研究得出体内与体外最佳的种子细胞混合共培养比例不同,在体内最佳混合共培养比率中间充质干细胞所占比率更高于体外,猜测这可能与间充质干细胞在体内创造了一个成软骨诱导的微环境有关。 共培养系统中,细胞混合比率在细胞间相互作用中扮演着重要的角色,影响间充质干细胞的分化和软骨样细胞外基质的分泌。由于种子细胞的活性、来源不同和培养环境的不同,导致最佳混合培养比率的不同。具有稳定的天然软骨细胞表型和细胞活性,有助于降低共培养系统中软骨细胞所占的比率和间充质干细胞的分化成软骨。未来的研究应该聚焦在减少软骨细胞的比率,提高间充质干细胞的比率,同时获得满意的新生软骨组织。 2.2.3 直接或间接共培养 直接共培养或间接共培养直接影响着共培养细胞的细胞间相互作用模式(图2),包括细胞-细胞间隙连接、细胞-细胞外基质、细胞-细胞因子或者它们之间联合模式。细胞间相互作用的模式也许影响着软骨细胞表型的维持和成软骨诱导的作用。间接共培养是机械地把共培养细胞在物理空间上分离,如Transwell小室[10],单独研究细胞分泌的细胞因子对另一种细胞生物学行为的作用。直接共培养是把共培养细胞相互均匀混合在一块,除了细胞因子对细胞产生作用外,还会通过细胞表面分子受体,细胞分泌的细胞外基质等方式参与到细胞间的相互作用中[37]。 间接共培养强调软骨细胞或间充质干细胞分泌因子对共培养细胞增殖、细胞外基质分泌和成软骨分化发挥重要作用。并且已经有报道称,在间接共培养系统中没有异种细胞间物理接触的前提下,检测到了软骨细胞分泌相关细胞因子,并成功的诱导了间充质干细胞分化成软骨[38-40]。然而,有研究称细胞间的物理接触也许扮演着主导作用[41-42],单独的依靠细胞分泌的细胞因子不能或极小程度的改变间充质干细胞成软骨基因的上调。通过整合蛋白、生长因子和细胞因子实现近距离的细胞间交流和信号传递发挥的作用不可忽视[43-44]。应用微球形式共培养的研究中发现,下调了间充质干细胞的X胶"
| [1] Filardo G, Perdisa F, Roffi A, et al.Stem cells in articular cartilage regeneration. J Orthop Surg Res. 2016;11:42.[2] Harris JD, Siston RA, Brophy RH, et al.Failures, re-operations, and complications after autologous chondrocyte implantation--a systematic review. Osteoarthritis Cartilage. 2011;19(7): 779-791.[3] Niemeyer P, Salzmann GM, Hirschmuller A, et al.[Factors that influence clinical outcome following autologous chondrocyte implantation for cartilage defects of the knee]. Z Orthop Unfall. 2012; 150(1):83-88.[4] Vasiliadis HS, Wasiak J, Salanti G.Autologous chondrocyte implantation for the treatment of cartilage lesions of the knee: a systematic review of randomized studies. Knee Surg. 2010; 18(12):1645-1655.[5] Nazempour A, Van Wie BJ. Chondrocytes, Mesenchymal Stem Cells, and Their Combination in Articular Cartilage Regenerative Medicine. Ann Biomed Eng. 2016; 44(5): 1325-1354.[6] Goers L, Freemont P, Polizzi KM.Co-culture systems and technologies: taking synthetic biology to the next level. J R Soc Interface. 2014;11(96). pii: 20140065. [7] Krinner A, Roeder I.Quantification and modeling of stem cell-niche interaction. Adv Exp Med Biol. 2014; 844:11-36.[8] Lawrence TS, Beers WH, Gilula NB.Transmission of hormonal stimulation by cell-to-cell communication. Nature. 1978; 272(5653):501-506.[9] Gayathri L, Dhanasekaran D, Akbarsha MA.Scientific concepts and applications of integrated discrete multiple organ co-culture technology. J Pharmacol Pharmacother. 2015;6(2):63-70.[10] Huang CP, Lu J, Seon H, et al. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab Chip. 2009; 9(12):1740-1748.[11] Li AP. In vitro evaluation of human xenobiotic toxicity: scientific concepts and the novel integrated discrete multiple cell co-culture (IdMOC) technology. Altex. 2008; 25(1):43-49.[12] Hendriks J, Riesle J, van Blitterswijk CA. Co-culture in cartilage tissue engineering. J Tissue Eng Reg Med. 2007; 1(3):170-178.[13] Wu L, Leijten JC, Georgi N, et al.Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17(9-10): 1425-1436.[14] Wu L, Prins HJ, Helder MN, et al.Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A. 2012;18(15-16):1542-1551.[15] Acharya C, Adesida A, Zajac P, et al.Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Phys. 2012;227(1):88-97.[16] Windt TS, Hendriks JA, Zhao X, et al.Concise review: unraveling stem cell cocultures in regenerative medicine: which cell interactions steer cartilage regeneration and how? Stem Cells Trans Med. 2014;3(6):723-733.[17] Meretoja VV, Dahlin RL, Kasper FK, et al.Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials. 2012;33(27): 6362-6369.[18] Yang YH, Lee AJ, Barabino GA.Coculture-driven mesenchymal stem cell-differentiated articular chondrocyte-like cells support neocartilage development. Stem Cells Trans Med. 2012;1(11):843-854.[19] Ouyang X, Wei B, Mao F, et al.Uncultured bone marrow mononuclear cells delay the dedifferentiation of unexpanded chondrocytes in pellet culture. Cell Tissue Res. 2015;361(3): 811-821.[20] Zhang F, Su K, Fang Y, et al.A mixed co-culture of mesenchymal stem cells and transgenic chondrocytes in alginate hydrogel for cartilage tissue engineering. J Tissue Eng Regen Med. 2015;9(1):77-84.[21] Vats A, Bielby RC, Tolley N, et al.Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Eng. 2006;12(6):1687-1697.[22] Pereira RC, Costa-Pinto AR, Frias AM, et al.In vitro chondrogenic commitment of human Wharton's jelly stem cells by co-culture with human articular chondrocytes. J Tissue Eng Regen Med. 2015.[23] Zheng P, Ju L, Jiang B, et al.Chondrogenic differentiation of human umbilical cord bloodderived mesenchymal stem cells by coculture with rabbit chondrocytes. Mol Med Reports. 2013; 8(4):1169-1182.[24] Wang L, Ott L, Seshareddy K, et al.Musculoskeletal tissue engineering with human umbilical cord mesenchymal stromal cells. Regen Med. 2011;6(1):95-109.[25] Chai DH, Arner EC, Griggs DW, et al.Alphav and beta1 integrins regulate dynamic compression-induced proteoglycan synthesis in 3D gel culture by distinct complementary pathways. Osteoarthritis Cartilage. 2010; 18(2): 249-256.[26] Diaz-Romero J, Gaillard JP, Grogan SP, et al.Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005; 202(3):731-742.[27] Kisiday JD, Jin M, DiMicco MA, et al.Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37(5): 595-604.[28] Sabatino MA, Santoro R, Gueven S, et al. Cartilage graft engineering by co-culturing primary human articular chondrocytes with human bone marrow stromal cells. J Tissue Eng Regen Med. 2015;9(12):1394-1403.[29] Taylor DW, Ahmed N, Hayes AJ, et al.Hyaline cartilage tissue is formed through the co-culture of passaged human chondrocytes and primary bovine chondrocytes. J Histochem Cytochem. 2012;60(8):576-587.[30] Lacombe-Gleize S, Gregoire M, Demignot S, et al.Implication of TGF beta 1 in co-culture of chondrocytes-osteoblasts. In Vitro Cell Dev Biol Anim. 1995;31(9): 649-652.[31] Schaefer D, Martin I, Shastri P, et al.In vitro generation of osteochondral composites. Biomaterials. 2000;21(24):2599- 2606.[32] Sanchez C, Deberg MA, Piccardi N, et al.Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2005; 13(11):988-997.[33] Spalazzi JP, Dionisio KL, Jiang J, et al.Osteoblast and chondrocyte interactions during coculture on scaffolds. IEEE Eng Med Biol Mag. 2003;22(5):27-34.[34] Jiang J, Nicoll SB, Lu HH.Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem Biophys Res Commun. 2005;338(2):762-770.[35] Qing C, Wei-ding C, Wei-min F.Co-culture of chondrocytes and bone marrow mesenchymal stem cells in vitro enhances the expression of cartilaginous extracellular matrix components. Brazil J Med Biol Res. 2011;44(4):303-310.[36] Mo XT, Guo SC, Xie HQ, et al.Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone. 2009;45(1):42-51.[37] Heng BC, Cao T, Lee EH.Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem cells (Dayton, Ohio). 2004;22(7):1152-1167.[38] Tan AR, Dong EY, Andry JP, et al.Coculture of engineered cartilage with primary chondrocytes induces expedited growth. Clin Orthop Relat Res. 2011;469(10):2735-2743.[39] Ahmed N, Dreier R, Gopferich A, et al.Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Phys Biochem. 2007;20(5):665-678.[40] Fischer J, Dickhut A, Rickert M, et al.Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62(9):2696-2706.[41] Richardson SM, Walker RV, Parker S, et al.Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells (Dayton, Ohio). 2006;24(3):707-716.[42] Zuo Q, Cui W, Liu F, et al.Co-cultivated mesenchymal stem cells support chondrocytic differentiation of articular chondrocytes. Int Orthop. 2013;37(4):747-752.[43] Djouad F, Delorme B, Maurice M, et al.Microenvironmental changes during differentiation of mesenchymal stem cells towards chondrocytes. Arthritis Res Ther. 2007;9(2):R33.[44] Varas L, Ohlsson LB, Honeth G, et al.Alpha10 integrin expression is up-regulated on fibroblast growth factor-2-treated mesenchymal stem cells with improved chondrogenic differentiation potential. Stem Cells Dev. 2007;16(6):965-978.[45] Bian L, Zhai DY, Mauck RL, et al. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17(7-8):1137-1145.[46] Battiston KG, Cheung JW, Jain D, et al.Biomaterials in co-culture systems: towards optimizing tissue integration and cell signaling within scaffolds. Biomaterials. 2014;35(15): 4465-4476.[47] Rowland CR, Colucci LA, Guilak F.Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds. Biomaterials. 2016;91: 57-72.[48] Shijun X, Junsheng M, Jianqun Z, et al. In vitro three-dimensional coculturing oly3-hydroxybutyrate-co-3- hydroxyhexanoate with mouse-induced pluripotent stem cells for myocardial patch application. J Biomater Appl. 2016;30(8): 1273-1282.[49] Kalpakci KN, Kim EJ, Athanasiou KA.Assessment of growth factor treatment on fibrochondrocyte and chondrocyte co-cultures for TMJ fibrocartilage engineering. Acta Biomater. 2011;7(4):1710-1718.[50] He X, Feng B, Huang C, et al.Electrospun gelatin/polycaprolactone nanofibrous membranes combined with a coculture of bone marrow stromal cells and chondrocytes for cartilage engineering. Int J Nanomed. 2015; 10:2089-2099.[51] Levorson EJ, Santoro M, Kasper FK, et al.Direct and indirect co-culture of chondrocytes and mesenchymal stem cells for the generation of polymer/extracellular matrix hybrid constructs. Acta Biomater. 2014;10(5):1824-1835.[52] Benya PD, Shaffer JD.Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215-224.[53] Darling EM, Athanasiou KA.Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23(2):425-432.[54] Mata-Miranda MM, Martinez-Martinez CM, Noriega-Gonzalez JE, et al.Morphological, genetic and phenotypic comparison between human articular chondrocytes and cultured chondrocytes. Histoch Cell Biol. 2016;146(2):183-189.[55] Kubosch EJ, Heidt E, Bernstein A, et al.The trans-well coculture of human synovial mesenchymal stem cells with chondrocytes leads to self-organization, chondrogenic differentiation, and secretion of TGFbeta. Stem Cell Res Ther. 2016;7(1):64.[56] Cooke ME, Allon AA, Cheng T, et al.Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthritis Cartilage. 2011;19(10):1210-1218.[57] Fong CY, Subramanian A, Gauthaman K, et al.Human umbilical cord Wharton's jelly stem cells undergo enhanced chondrogenic differentiation when grown on nanofibrous scaffolds and in a sequential two-stage culture medium environment. Stem Cell Rev. 2012;8(1): 195-209.[58] Armstrong JP, Shakur R, Horne JP, et al.Artificial membrane-binding proteins stimulate oxygenation of stem cells during engineering of large cartilage tissue. Naturecommunications. 2015;6:7405.[59] Kalpakci KN, Kim KJ, Athanasiou KA. Assessment of Growth Factor Treatment on Fibrochondrocyte,Chondrocyte Co-Cultures for TMJ Fibrocartilage Engineering. Acta Biomater. 2011; 7(4): 1710-1718.[60] 张勇,赵建宁,韩宁波.不同培养条件下脂肪干细胞与软骨细胞共培养的研究[J].中国矫形外科杂志,2009,17(10):782-785. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [5] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [6] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [7] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [8] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [9] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [10] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [11] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [12] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [13] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| [14] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [15] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||