Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (11): 1783-1789.doi: 10.3969/j.issn.2095-4344.2017.11.025
Previous Articles Next Articles
Research progress of classification and pathogenesis in ankylosing spondylitis animal models
Tan Xi1, Xu Yong-yue1, Qiu Dong-ni1, Huang Run-yue1, 2, He Yi-ting1, 3
- 1Second Institute of Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 2Guangdong Provincial Academy of Chinese Medical Sciences, Guangzhou 510006, Guangdong Province, China; 3New Drug Development Center of Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou 510120, Guangdong Province, China
-
Revised:2016-12-06Online:2017-04-18Published:2017-05-06 -
Contact:He Yi-ting, M.D., Professor, Second Institute of Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; New Drug Development Center of Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou 510120, Guangdong Province, China -
About author:Tan Xi, Studying for master’s degree, Second Institute of Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China -
Supported by:the National Natural Science Foundation of China, No. 81273736, 81673898
CLC Number:
Cite this article
Tan Xi, Xu Yong-yue, Qiu Dong-ni, Huang Run-yue, He Yi-ting. Research progress of classification and pathogenesis in ankylosing spondylitis animal models[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(11): 1783-1789.
share this article

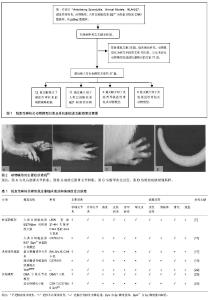
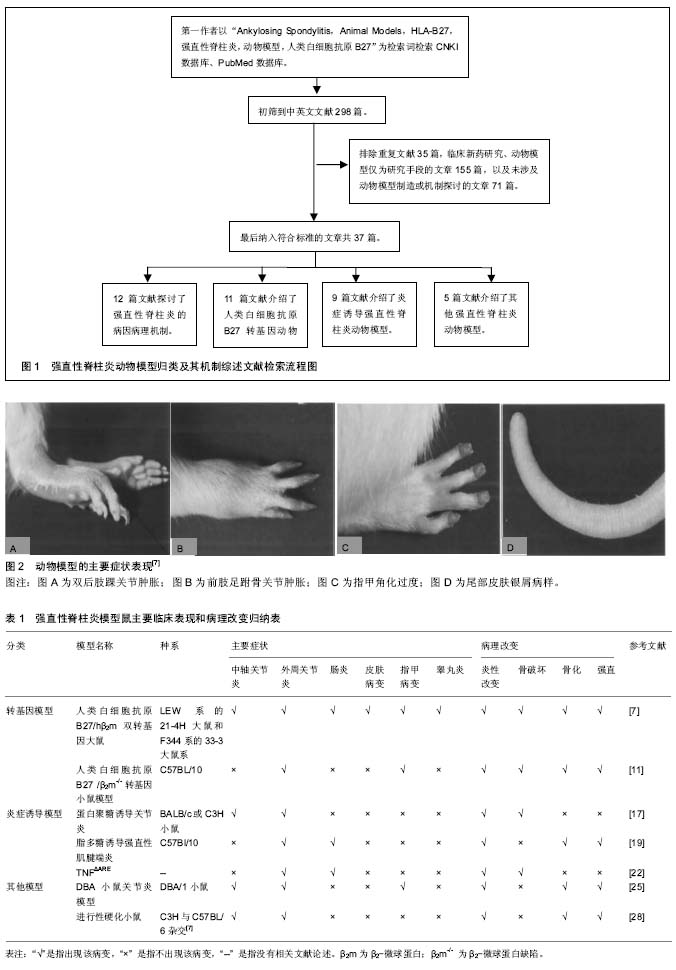
人类白细胞抗原B27转基因动物模型的运用,使强直性脊柱炎及其相关疾病的研究取得重大的突破,但是也存在很多不足,如成本高、表达不稳定、阳性率低。并且人类白细胞抗原B27分子在强直性脊柱炎及其相关疾病的发病机制仍然不明确。其他非转基因动物模型也与脊柱关节炎的表现极为相似,亦可能成为研究此类疾病的不同路径。 2.1 人类白细胞抗原B27转基因动物模型 流行病学调查证实在强直性脊柱炎患者中人类白细胞抗原B27阳性率高达90%-95%[5]。人类白细胞抗原B27基因是人类主要组织相容性复合物(major histocompatibility complex,MHC)Ⅰ类分子B位点上的等位基因,位于人的第6号染色体的短臂上,由8个外显子和7个内含子构成。1987年Krimpenfort等[6]最早建立人类白细胞抗原B27转基因小鼠模型,证实了人类白细胞抗原B27的遗传易感性,随后该模型鼠被广泛运用于脊柱关节炎的实验研究和新药研制。在脊柱关节炎中,强直性脊柱炎是与人类白细胞抗原具有最强关联性的疾病,鉴于人类白细胞抗原B27在强直性脊柱炎发病中的重要作用,下面具体阐述两种人类白细胞抗原B27转基因动物模型。 2.1.1 人类白细胞抗原B27/人β2-微球蛋白双转基因大鼠(HLA-B27/human β2m transgenic rat)模型 造模方法:运用显微注射法在LEW系大鼠受精卵内转入含人类白细胞抗原-B*2705基因的6.5 kb的EcoRⅠ片段和含15 kb Sall-Pvul 片段的人β2-微球蛋白 基因,模型鼠出生后从其尾部活检分离染色体DNA并做southern印迹杂交法鉴定[7]。研究证明LEW系的21-4H鼠和F344系的33-3鼠系具有更明显的易感性[7]。 主要症状:骶髂关节炎,双后肢关节炎,胃肠道炎症,银屑病样皮肤,指甲损害(角化过多、营养不良),雄性睾丸炎,少数伴有葡萄膜炎及心脏受累[7]。值得注意的是,该双转基因大鼠在无特定病原体(SPF)环境中并不表现出关节炎的症状,当移至普通环境时才发病[8]。 目前就人类白细胞抗原B27在强直性脊柱炎中的发病机制主要有3个假说:①假说一:人类白细胞抗原B27异常形式的免疫识别。人类白细胞抗原B27是属于Ⅰ类主要组织相容性复合物抗原,其重要作用为抗原递呈多肽给细胞毒性CD8+T细胞,在强直性脊柱炎疾病中表现为对自身抗原发生免疫性反应;②假说二:未折叠蛋白反应。人类白细胞抗原B27分子在内质网中相比其他的主要组织相容性复合物Ⅰ类分子处理进程更缓慢,在γ-干扰素或/和肿瘤坏死因子α的刺激下,可能会导致未折叠分子的上调和未折叠蛋白反应标志物的增加,此过程与内质网应激有关[9];③假说三:重链二聚体异常增加,人类白细胞抗原B27分子通过二硫化物的相互作用形成重链二聚体,此二聚体通过模式识别受体的方式被识别,从而诱导自发炎症性反应[10]。 特点:①优点:该模型表现出非常全面的临床症状,与强直性脊柱炎的临床表现极为相似;②缺点:其发病机制仍不能完全阐明。该模型制造周期长,模型鼠至老龄期,症状轻,与临床强直性脊柱炎有别。且其技术要求严格,操作步骤繁琐,价格昂贵。 2.1.2 人类白细胞抗原B27/β2-微球蛋白缺陷转基因小鼠(HLA-B27/β2m-/- transgenic mice)模型 造模方法:是在C57BL/10小鼠的基因背景下,转基因人类白细胞抗原B27小鼠与β2m-/-小鼠杂交,其中人类白细胞抗原B27阳性的第2代鼠互相杂交,建立人类白细胞抗原B27 β2m-/- tgmice[8]。用PCR去鉴定人类白细胞抗原B27转基因和纯合子突变的β2-微球蛋白。 主要症状:4-8个月出现肌腱端炎、关节炎和指甲改变,伴有毛发脱落,随后脚踝和足跗骨关节进行性僵硬,且雄性好发,起病早,症状较雌性严重。该小鼠模型未累及脊柱和骶髂关节、眼和其他脏器。组织病理表现在脚踝和足跗骨关节连接部位有纤维蛋白渗出和白细胞浸润,刺激软骨细胞增殖分化,骨桥形成,关节软骨融合,最终出现强直。然而这种骨桥的形成主要集中在软骨的边缘处,中心软骨在很长一段时间保持完整[11-12]。与人类白细胞抗原B27/β2-微球蛋白双转基因大鼠类似,该转基因小鼠在SPF级环境下不发生病变,只用当动物移至普通环境下2-4周内出现指甲和关节的改变[8]。 可能的机制:在主要组织相容性复合体表达的过程中,人类白细胞抗原重链段在细胞内质网中与β2微球蛋白和肽类结合,形成三聚体转运到细胞膜表面,β2微球蛋白缺陷的小鼠不表达Ⅰ类分子在细胞膜表面,由于主要组织相容性复合物Ⅰ类分子参与的阳性选择减少,CD8+T细胞随之减少[13]。而在强直性脊柱炎患者病变的滑膜关节中可见CD8+T细胞、CD4+T细胞同时大量浸润,学者猜测可能是由于人类白细胞抗原B27基因的过量表达和β2-微球蛋白的缺乏,这种比例的失衡使Ⅰ类分子大量滞留于内质网细胞质中,这种情况人类白细胞抗原B27提呈细胞外的多肽或者导致蛋白的降解,降解产物将成为自身抗原,被Ⅱ类分子提呈给CD4+细胞,从而诱发疾病[14-15]。 特点:①优点:β2微球蛋白缺陷转基因小鼠模型表现为外周关节病变、进行性僵硬、趾炎和毛发脱落。雄性发病高于雌性,与强直性脊柱炎部分病变相似;②缺点:主要是外周关节的病变,未累及中轴关节、眼及其他脏器,与强直性脊柱炎有异。 2.2 炎症诱导强直性脊柱炎动物模型 2.2.1 蛋白聚糖诱导关节炎小鼠模型 造模方法:蛋白聚糖是从关节软骨、椎间盘髓核等组织分离出的一种细胞外基质蛋白,蛋白聚糖诱导关节炎模型是指将蛋白聚糖反复免疫BALB/c或C3H品系小鼠,使其出现肌腱端炎和脊柱炎。在第1次免疫后一二周之内,大部分蛋白聚糖诱导关节炎小鼠就会出现外周关节炎的症状,表现为四肢足趾部红肿,12周后外周关节炎的症状出现率大于98%,中轴关节出现症状最早在第14周。该小鼠模型中可发展为蛋白聚糖诱导脊柱关节炎,类似于脊柱关节炎,此动物模型疾病的进展与临床强直性脊柱炎的表现极为相似,同样是首先出现骶髂关节肌腱端炎,然后沿脊柱向上发展,侵犯多个椎间盘。 主要症状:表现为包括脊柱炎在内的多关节滑膜炎,进行性的关节软骨和骨侵蚀,导致动关节的强直和畸形,病理组织学改变为脊柱纤维环内单核细胞浸润,软骨细胞增殖,其特征性的病理改变使蛋白聚糖诱导关节炎模型在强直性脊柱炎研究中有独特的作用[4,16]。 主要机制:软骨是无血管组织,它不受免疫系统监视,当软骨受到外来因素、蛋白酶损伤降解后会暴露出特定的抗原表位,可被自身免疫系统识别而诱发特定的免疫反应[16]。蛋白聚糖是关节炎软骨的主要组成部分之一,其中的G1域多肽会诱导T细胞募集,发生细胞免疫反应,从而造成类似强直性脊柱炎的炎症损伤[17-18]。 特点:①优点:操作简便,周期短,症状明显;②缺点:强直性脊柱炎是与人类白细胞抗原B27密切相关的疾病。该模型表现出更少的基因限制性,与之有别。 2.2.2 脂多糖诱导小鼠强直性肌腱端炎模型 造模方法:是以强直性肌腱端炎易感的C57Bl/10小鼠为基因背景,反复多次腹腔注射脂多糖免疫[19]。 主要症状:踝关节红肿,随之后爪、踝和足跗关节进行性僵硬,伴有肠炎。病理表现有关节周围有炎性细胞浸润,随后软骨转化,骨赘形成,逐渐关节僵直[19]。 可能的机制:脂多糖是革兰阴性细菌细胞膜壁上一种成分,具有抗原性,可以有效刺激免疫炎症反应,诱导多种炎性细胞因子、趋化因子及黏附分子的在关节局部聚集,引起对自身蛋白聚糖的免疫反应[19]。脂多糖诱发固有免疫应答可能只存在造模的前期,造模中后期关节炎的发病率逐渐持平。 特点:①优点:此小鼠在一定的环境下可自发的出现起止点炎、关节强直的病理表现,与人类脊柱关节炎有许多相似之处。脂多糖诱导后小鼠可出现肠道炎症和后爪部肌腱端症;②缺点:诱导周期较长,成功率低;在无菌环境下肠道炎症和肌腱端炎症均无法诱导。 2.2.3 TNFΔARE鼠动物模型 造模方法:ARE是一类包涵AUUUA五核苷酸的腺苷酸、尿嘧啶多聚体的基因序列,具有调节mRNA稳定性的功能[20]。TNFΔARE模型的造模机制为敲除鼠染色体肿瘤坏死因子中ARE(AU-rich elements),使肿瘤坏死因子mRNA反转录调节缺陷,导致造血和间质组织中肿瘤坏死因子mRNA水平上升,使得内源性的肿瘤坏死因子的表达不断增加[21]。 主要症状:该模型发展为类似于人脊柱关节炎的疾病,例如脊柱和骶髂关节炎、外周关节炎、肠炎和肌腱端炎。表现为关节肿胀,前后足爪形态畸形,行动困难。组织器官上的改变有胸腺萎缩,但是流式细胞仪检测在胸腺和外周血中没有发现T细胞的波动[22]。 主要机制:临床上强直性脊柱炎可见骶髂关节肿瘤坏死因子α富集表达, 肿瘤坏死因子α作为重要的促炎因子,可与白细胞介素1、白细胞介素6等细胞因子构成局部炎症微环境,触发一系列免疫炎症反应[23]。TNFΔARE模型证实了肿瘤坏死因子在强直性脊柱炎中的重要致病作用。大量研究证明肿瘤坏死因子抑制剂能有效改善疼痛和关节僵硬的症状[23],然而对疾病影像学上进展的影响一直存在争议,有研究发现肿瘤坏死因子抑制剂不能延缓或阻止关节骨赘形成和强直[24]。炎症和新骨形成之间的确切关系还需进一步的研究证明。 特点:①优点:TNFΔARE鼠动物模型是类似于人脊柱关节炎疾病的动物模型,有关节炎和肠炎的症状;②缺点:操作技术要求高,造价高昂,难以推广。 2.3 其他强直性脊柱炎动物模型 2.3.1 DBA/1小鼠关节炎模型 造模方法:DBA/1小鼠关节炎模型会随着年龄的增加至21周龄时逐渐自发出现关节炎,总体发病率约为26.7%[25]。起初,DBA/1小鼠关节炎模型是被认为是类风湿性关节炎的动物模型,后来研究发现该模型具有与脊柱关节炎极为相似的病理表现。 主要症状:肌腱端炎,趾炎,滑膜炎、软骨下骨破坏,附着点成纤维细胞增殖、异位软骨和骨形成导致骨桥形成关节强直[25-26]。 主要机制:DBA/1小鼠是国际公认的筛选和研究治疗关节炎的动物模型,在DBA/1小鼠关节炎模型中,骨形态蛋白信号通路是关键的信号转导通路,头蛋白是一种参与脊索形成的蛋白,能特异性的与形态蛋白结合,从而抑制骨形态蛋白信号转导。有学者认为,骨形态蛋白拮抗剂能有效抑制骨赘形成和关节进行性强直,或许能成为强直性脊柱炎治疗的互补的治疗手段[26-27]。 特点:①优点:是以DBA/1小鼠为基因背景,自发性的模型鼠。造模方法简便且有一定的成功率;②缺点:周期长。 2.3.2 进行性硬化小鼠模型 简介:行性硬化小鼠模型是一种与人类主要组织相容性复合物Ⅰ类分子无关联的遗传性疾病,是小鼠常染色体ank/ank单基因的隐性缺失[28]。 主要症状:起初有四肢关节的红肿,随后中轴和外周关节进行性强直。病理改变为起初外周关节有中性粒细胞和巨噬细胞浸润,随后软骨转化,骨赘形成,逐渐关节僵直。 主要机制:Ank基因编码无机焦磷酸盐转运体,由于ank基因功能的丧失,细胞内的焦磷酸盐增加,而细胞外的焦磷酸盐下降,导致钙羟磷灰石的沉积、软骨或椎间盘钙化进而导致关节或脊柱强直,出现影响学的“竹节样”改变[11,18,28]。尽管模型鼠的X射线改变与强直性脊柱炎非常相似,但是这种强直是非炎症性的,与强直性脊柱炎疾病有异[29]。 特点:①优点:可出现中轴关节和外周关节的强直,关节周围软组织纤维化、骨化和关节强直该模型鼠与人强直性脊柱炎的X射线变化非常相似;②缺点:ank/ank基因纯合子鼠因常染色体隐形异常,致鼠自发性进行性强直,至今这个基因未被鉴定出来,产物也不清楚。这些小鼠经过大量免疫处理,不能用来说明此类疾病所涉及的免疫因素。"

| [1] Robinson WP, Van Der Linden SM, Khan MA, et al. HLA–Bw60 increases susceptibility to ankylosing spondylitis in HLA–B27+ patients. Arthritis Rheum. 1989;32(9): 1135-1141.[2] Nahal SR, Richard CM, Barthelot JM, et al. The familial form of spondyloarthropathy: a clinical study of 115 multiplex families. Arthritis Rheum. 2000;43:1356-1365.[3] Mikecz K, Glant TT, Poole AR. Immunity to cartilage proteoglycans in balb/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheumatol. 1987, 30(3):306–318.[4] Martínez A, Pachecotena C, Vázquezmellado J, et al. Relationship between disease activity and infection in patients with spondyloarthropathies. Ann Rheum Dis. 2004;63(10): 1338-1340.[5] 陈慰峰.医学免疫学[M].北京:人民卫生出版社,2004:53-63. [6] Krimpenfort P, Rudenko G, Hochstenbach F, et al. Crosses of two independently derived transgenic mice demonstrate functional complementation of the genes encoding heavy (HLA-B27) and light (beta 2-microglobulin) chains of HLA class I antigens. Embo J. 1987; 6(6):1673-1676.[7] Hammer RE, Maika SD, Richardson JA, et al. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and humanβ 2 m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63(5): 1099-1112.[8] Khare SD, Luthra HS, David CS. Spontaneous inflammatory arthritis in HLA-B27 transgenic mice lacking beta 2-microglobulin: a model of human spondyloarthropathies.J Exp Med. 1995;182(4): 1153-1158.[9] Dangoria NS, DeLay ML, Kingsbury DJ, et al. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J Biol Chem. 2002;277(26): 23459-23468.[10] Kollnberger S, Bird LA, Roddis M, et al. HLA-B27 heavy chain homodimers are expressed in HLA-B27 transgenic rodent models of spondyloarthritis and are ligands for paired Ig-like receptors. J Immunol. 2004;173(3): 1699-1710.[11] Zhang Y, Shi S, Ciurli C, et al. Animal models of ankylosing spondylitis.Curr Rheum Rep. 2002;4(6): 507-512.[12] [12]Weinreich S, Eulderink F, Capkova J, et al. HLA-B27 as a relative risk factor in ankylosing enthesopathy in transgenic mice. Human Immunol. 1995;42(2): 103-115.[13] Allen H, Fraser J, Flyer D, et al. Beta 2-microglobulin is not required for cell surface expression of the murine class I histocompatibility antigen H-2Db or of a truncated H-2Db. Proc Nat Acad Sci. 1986;83(19): 7447-7451.[14] Taurog JD, Maika SD, Simmons WA, et al. Susceptibility to inflammatory disease in HLA-B27 transgenic rat lines correlates with the level of B27 expression. J Immunol. 1993; 150(9):4168-4178.78.[15] Rosenbaum JT, Davey MP. Time for a gut check: Evidence for the hypothesis that HLA–B27 predisposes to ankylosing spondylitis by altering the microbiome. Arthritis Rheum.2011; 63(11):3195-3198.[16] Bardos T, Szabo Z, Czipri M, et al. A longitudinal study on an autoimmune murine model of ankylosing spondylitis. Ann Rheum Dis. 2005;64(7): 981-987.[17] Zhang Y, Guerassimov A, Leroux J, et al. Arthritis induced by proteoglycan aggrecan G1 domain in BALB/c mice. Evidence for t cell involvement and the immunosuppressive influence of keratan sulfate on recognition of t and b cell epitopes. J Clin Invest. 1998;101(8):1678-1686.[18] Adarichev VA, Glant TT. Experimental spondyloarthropathies: animal models of ankylosing spondylitis. Curr Rheumatol Rep. 2006;8(4): 267-274.[19] Capkova J, Hrncir T, Kubatova A, et al. Lipopolysaccharide treatment suppresses spontaneously developing ankylosing enthesopathy in B10. BR male mice: The potential role of interleukin-10. BMC Musculoskelet Disord. 2012;13(1): 110.[20] Kontoyiannis D, Pasparakis M, Pizarro TT, et al. Impaired On/Off Regulation of TNF Biosynthesis in Mice Lacking TNF AU-Rich Elements : Implications for Joint and Gut-Associated Immunopathologies. Immunity. 1999;10(3):387-398.[21] Braem K, Lories RJ. Insights into the pathophysiology of ankylosing spondylitis: contributions from animal models. Joint Bone Spine. 2012;79(3): 243-248.[22] Kontoyiannis D, Pasparakis M, Pizarro TT, et al. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10(3): 387-398.[23] Zhang JR, Liu XJ, Xu WD, et al. Effects of tumor necrosis factor-α inhibitors on new bone formation in ankylosing spondylitis. Joint Bone Spine. 2015;83(3):257-264.[24] Machado MA, Barbosa MM, Almeida AM, et al. Treatment of ankylosing spondylitis with TNF blockers: a meta-analysis. Rheumatol Int. 2013;33(9):2199-2213.[25] Lories RJ, Matthys P, De Vlam K, et al. Ankylosing enthesitis, dactylitis, and onychoperiostitis in male DBA/1 mice: a model of psoriatic arthritis. Ann Rheum Dis. 2004;63(5): 595-598.[26] Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005; 115(6): 1571-1579.[27] Waite KA, Eng C. From developmental disorder to heritable cancer: it's all in the BMP/TGF-β family. Nat Rev Genet. 2003;4(10):763-773.[28] Krug HE, Taurog JD. HLA-B27has no effect on the phenotypic expression of progressive ankylosis in ank/ank mice. J Rheumatol. 2000;27(5): 1257-1259.[29] 李维, 吴强. HLA-B27 亚型及其与强直性脊柱炎关系的研究进展[J]. 免疫学杂志, 2002, 18(B06): 191-194.[30] Jobanputra P, Choy EH, Kingsley GH, et al. Cellular immunity to cartilage proteoglycans: relevance to the pathogenesis of ankylosing spondylitis. Ann Rheum Dis. 1992;51(8): 959-962.[31] Zou J, Zhang Y, Thiel A, et al. Predominant cellular immune response to the cartilage autoantigenic G1 aggrecan in ankylosing spondylitis and rheumatoid arthritis. Rheumatology. 2003;42(7): 846-855.[32] Sung IH, Kim TH, Bang SY, et al. IL-23R polymorphisms in patients with ankylosing spondylitis in Korea. J Rheumatol. 2009;36(5): 1003-1005.[33] 王昊, 阎小萍, 孔维萍,等.补肾强督方对强直性脊柱炎患者骨质疏松及骨量减少的影响[J].中国中西医结合杂志, 2011,31(4): 471-474.[34] Glatigny S, Fert I, Blaton MA, et al. Proinflammatory T helper 17 cells are expanded and induced by dendritic cells in spondyloarthritis-prone HLA-B27 transgenic rat. Arthritis Rheum. 2012;64(1):110-120.[35] Zeng L,Lindstrom MJ.Ankylosing spondylitis macrophage production of higher levels of interleukin-23 in response to lipopolysaccharide without induction of a significant unfolded protein response.Arthritis Rheum. 2011;63:3807-3817.[36] 刘毅,蔡醒华. 肠道炎症及细菌感染在强直性脊柱炎发病中的作用[J].中华内科杂志,1995,34(5): 337-338.[37] Braem K, Lories RJ. Insights into the pathophysiology of ankylosing spondylitis: contributions from animal models. Joint Bone Spine. 2012;79(3): 243-248. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [5] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [6] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [7] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [8] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [9] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [10] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [11] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Li Xuan, Sun Yimin, Li Longbiao, Wang Zhenming, Yang Jing, Wang Chenglin, Ye Ling. Manufacturing of nano-modified polycaprolactone microspheres and its biological effects in dental pulp cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1530-1536. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||