Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (10): 1623-1628.doi: 10.3969/j.issn.2095-4344.2017.10.024
Previous Articles Next Articles
Latest research on zirconia implant surface treatment
Xie Liu-rong, Wu Xiu-tuan, Li Wen-liang, Liao Hong-bing
- Department of Prosthodontics, College of Stamotology, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China
-
Received:2017-03-02Online:2017-04-08Published:2017-05-08 -
Contact:Liao Hong-bing, M.D., Professor, Department of Prosthodontics, College of Stamotology, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:Xie Liu-rong, Studying for master’s degree, Department of Prosthodontics, College of Stamotology, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 81260170; the Natural Science Foundation of Guangxi Zhuang Autonomous Region, No. 604197919071
CLC Number:
Cite this article
Xie Liu-rong, Wu Xiu-tuan, Li Wen-liang, Liao Hong-bing. Latest research on zirconia implant surface treatment[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(10): 1623-1628.
share this article
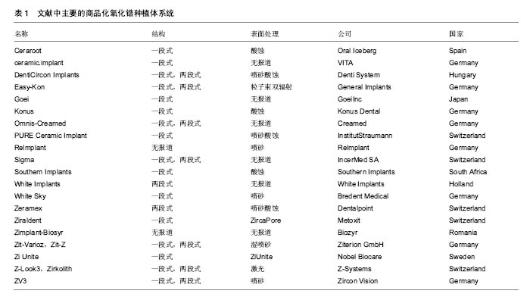
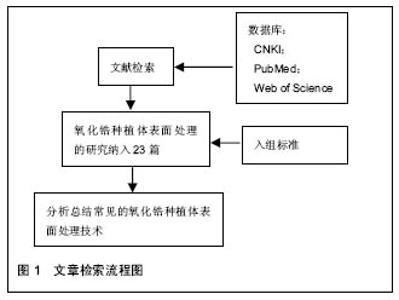
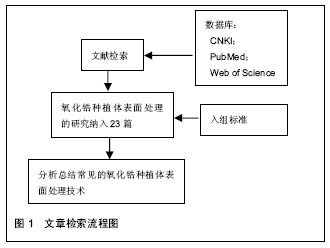
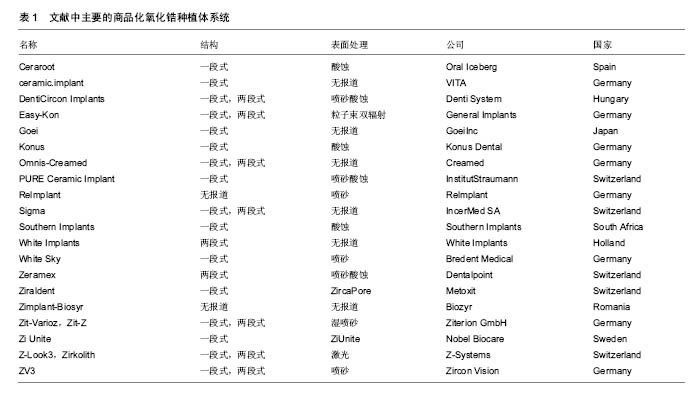
2.2 纳入资料的研究结果特征 2.2.1 表面处理技术 目前,常见的氧化锆种植体的表面处理技术有非涂层法和涂层法,非涂层法有物理法(喷砂、激光处理及紫外线处理等)、化学法(酸蚀)及物理化学法(喷砂酸蚀及选择性渗透酸蚀等);涂层法主要有表面涂层处理等。文章就上述方法,分别表述如下。 非涂层法: (1)喷砂:是将坚硬材料高速喷射到种植体表面,使种植体获得凹凸不平的粗糙面,增大表面积及表面自由能,以利于细胞和蛋白的黏附,促进骨结合的表面处理方式。目前,用于氧化锆种植体的喷砂材料主要有直径为25-250 μm的Al2O3和氧化锆颗粒。颗粒大小、电压强度、喷射速度及喷射时间等因素直接影响种植体表面的粗糙度,通过控制这些因素可以高效地获得理想的粗糙度。 根据Albrektsson和Wennerberg的研究,种植体的表面粗糙度(Surface Roughness,Sa)可以分为4种:光滑表面Sa < 0.5 μm;略粗糙表面0.5 μm≤Sa <1 μm;适度粗糙表面1 μm ≤ Sa < 2 μm;粗糙表面Sa > 2 μm,而适度粗糙和粗糙表面的种植体的骨整合要优于光滑和略粗糙的[6]。Mueller等[7]在研究种植体的设计和表面形貌对氧化锆种植体骨整合影响的动物实验中,发现喷砂后适度粗糙表面的氧化锆种植体-骨结合率(Bone to Implant Contact,BIC)高于光滑表面和粗糙表面的,其与喷砂酸蚀后的钛种植体的BIC无明显差异。Gahlert等[8]比较了两种不同表面形貌的氧化锆种植体和钛种植体的骨结合,术后8,12周,喷砂处理的氧化锆种植体(Sa=0.56 μm)的旋出扭矩(RemoveTorque,RTQ)均比常规切削的(Sa=0.13 μm)大,但两种氧化锆种植体的RTQ都显著小于喷砂酸蚀的钛种植体的(Sa=1.15 μm)。 喷砂的优点是成本低,但存在2个缺点,一是喷砂颗粒易残留在表面,造成污染;二是喷砂会导致种植体表面产生微裂纹。微裂纹产生后,在局部张应力下裂纹尖端的四方多晶体转变为单斜相晶体。喷砂对种植体强度的影响取决于压缩应力层和表面微裂纹的深度,当压缩应力层的深度不小于微裂纹的深度时,喷砂具有增韧效果;反之,喷砂会降低强度[9]。另有学者认为烧结前喷砂可降低喷砂对氧化锆机械性能的不利影响[10]。 (2)酸蚀:是用酸蚀剂蚀刻材料表面,消除表面部分材料和杂质,增大表面积,形成形态规则、均一的微观表面的常用技术。以前的研究认为氧化锆具有高化学惰性,它几乎不含硅,因此不能被酸蚀。但有文献显示氧化锆不仅能在加热时被氢氟酸(hydrofluoric acid,HF)、硫酸及硝酸等溶液酸蚀[11],且室温下能被氢氟酸酸蚀出粗糙面。 Flamant等[12]首次报道了氢氟酸酸蚀氧化钇稳定的四方相氧化锆的研究。该研究发现在室温下采用体积分数40%的氢氟酸酸蚀1 h,能使样品表面发生化学变化,形成粗糙面。该研究报道,氢氟酸的F能部分溶解表面氧化锆及其含有的稳定剂氧化钇,形成含氟、钇、锆的氟化物、氧化物和氢氧化物复合物。由于钇复合物(YF3)溶解度很低,一开始就析出在样品表面,形成八面体结晶。因锆复合物部分溶解,一定时间后,该复合物以粘结层样的结构也析出于样品表面。由此,氧化锆表面由光滑变粗糙。目前以上复合物对人体的影响仍不清楚,所以酸蚀后的彻底清洁很重要。 Gahlert等[13]报道在小型猪实验中,氢氟酸处理的氧化锆种植体的BIC与喷砂酸蚀处理的钛种植体的相似。但关于氢氟酸酸蚀的浓度、作用时间和温度的最优参数,现尚无报道。 (3)喷砂酸蚀:酸蚀多与喷砂技术相结合,称为喷砂酸蚀。喷砂酸蚀是种植体最常用的表面处理技术之一。喷砂酸蚀后的结构为在喷砂后形成的不规则大起伏中,有无数酸蚀刻形成的微孔,即为两个层次以上的二级窝洞的粗糙多孔结构。Zhao等[14]认为,喷砂酸蚀结构的一级孔洞为细胞提供附着点,引导细胞黏附、伸展和分化;二级微孔为细胞提供点状接触,刺激细胞反应,两者作用叠加则增加黏着斑水平从而促进骨源性环境形成。 Hirano等[15]分别用氧化锆、钛材料与人间充质干细胞共培养,发现喷砂酸蚀处理的氧化锆表面细胞的增殖能力、碱性磷酸酶活性和Runx2基因表达均高于喷砂处理的氧化锆及喷砂酸蚀处理的钛,电镜下见前者的表面在较大起伏中存在纳米级的纹理,其表面纹理较后两者的更精细,可能更适合细胞增殖和成骨向分化。Bergemann等[16]亦发现喷砂酸蚀处理的氧化锆表面,人成骨细胞增殖能力和骨钙素表达均优于喷砂处理组的。Schliephake等[17]在体内实验中发现喷砂酸蚀组的氧化锆种植体的BIC优于喷砂处理组的,且与喷砂酸蚀的钛种植体无明显差异。以上实验结果显示氧化锆种植体在同等条件下,喷砂酸蚀处理表现出优于单纯喷砂处理的趋势。 (4)选择性渗透酸蚀:是一种能将氧化锆表面变成高活性纳米多孔表面的新处理技术。它是在氧化锆表面覆一层特殊的渗透性玻璃并加热至玻璃的熔点,熔融的玻璃在氧化锆的晶界之间扩散产生的表面张力和毛细管力会引起表面晶粒的滑动、分离和重排。冷却至室温后,将其置于酸液中,玻璃被酸蚀,从而暴露出晶粒间新形成的纳米级多孔结构[18]。该技术只溶解材料表面的晶粒,且通过所覆的玻璃成分、加热的温度和时间可以改变孔隙的结构和分布,所以是选择性的。 Aboushelib最先提出了SIE技术,目的是增加氧化锆与树脂水门汀间的黏接力,结果发现它具有增大组织面粗糙度、提高粘接强度的效果。随后的研究还发现SIE处理氧化锆种植体表面可促进骨结合[18],SIE因而被应用于氧化锆种植体表面处理。 (5)激光处理:现今,激光已迅猛地发展于多个领域,特别是材料加工方面,激光被广泛地应用于材料表面处理,激光上釉、激光重熔、激光合金化、激光涂敷等,具有准确干洁、效率高、可控性强等优点。激光处理在氧化锆种植体表面处理技术中是一项新的尝试。激光处理氧化锆种植体表面的优点,一是种植体和激光仪器间不存在接触,可避免如喷砂酸蚀等处理带来的表面污染;二是可产生蜂窝小孔,增大表面粗糙度,更利于成骨细胞黏附;三是可增加种植体表面润湿性和含氧量,有利于获得更好的初期稳定性和骨结合。目前,处理氧化锆种植体表面的激光包括光纤激光、CO2激光[19,20]、飞秒激光及YAG激光等[21]。 光纤激光是指用掺稀土元素(如镱等)玻璃光纤作为增益介质的激光器所产生的激光。光纤激光光束稳定,没有空间波动,且波长较短(1 000-1 100 nm),能产生高能量光束。Kimie Yasuno等[22]发现光纤激光可以在氧化锆种植体表面形成有无数约2 μm宽沟的粗糙表面,且对纳米结构材料几乎无不利影响。Kimie Yasuno等[22]在用光纤激光处理氧化锆种植体表面的动物实验中,发现经光纤激光处理的种植体表面的平均BIC约是对照组(表面光滑)的4.2倍,前者的平均RTQ约是后者的2.4倍;这表明光纤激光处理氧化锆种植体表面可以促进骨结合。Taniguchi等[23]报道光纤激光处理的氧化锆表面,大鼠成骨样细胞系MC3T3-E1细胞繁殖能力、碱性磷酸酶活性和骨钙素mRNA表达水平均显著高于对照组。该处理能显著地增加动物体内氧化锆种植体的BIC和RTQ[23]。 CO2激光是一种气体激光,波长为10.6 μm,频率低、光子能量小,属于热加工,主要用于切割、表面处理等。研究发现它能提高氧化锆表面润湿性,增大表面能,促进人胚成骨细胞的黏附[19]。低功率CO2激光的处理可有效抑制氧化锆表面及悬液中的血链球菌和牙龈卟啉单胞菌的生长[20]。这表明CO2激光具有预防和治疗氧化锆种植体周围炎的潜力。 飞秒激光是一种脉冲激光,具有超短持续时间和超高瞬时功率,较传统的处理方式更精确、对表面的损伤更小,产生的粗糙表面拥有良好的骨结合[24]。 YAG激光是一种固体激光,但不同于一般的固体激光如红宝石激光、蓝宝石激光,它的发光晶体是石榴石,常用波长为1 064 nm,该激光按输出形式可分为连续式激光和脉冲式激光。Kae Kakura等[21]在体内实验中发现YAG激光辐照表面处理的氧化锆种植体的骨结合率和旋出扭矩均显著性地高于对照组,YAG激光辐照种植体表面能促进骨结合。但Kimie Yasuno等[22]指出YAG激光辐照会损坏种植体的表面螺纹,不建议YAG激光用于临床种植体的表面处理。 (6)紫外线处理:紫外线处理种植体表面能改变其理化特性,形成高亲水表面,从而有利于细胞的粘附和增殖[25],是一种能有效改善氧化锆表面润湿性的方法。Vogler等[26]认为材料表面接触角大于65°时为疏水性表面,小于65°时为亲水性表面。Yang等[27]用经紫外线照射的氧化锆材料与人牙龈成纤维细胞共培养,发现该处理能减小粗糙组和光滑组的表面接触角,提高材料的亲水性,显著促进了粗糙组表面成纤维细胞的增殖和分化。这给种植体与软组织接触区的表面处理提供了参考。Tuna等[28]证实了紫外线预处理氧化锆表面能改变材料表面的物理化学特性,促进细胞的黏附。紫外线处理操作简便,经济有效,可与其他处理相结合,以促进骨结合。 涂层法:表面涂层处理是采取不同技术,如浸渍、涂浆、等离子喷涂、溶胶-凝胶等,将涂层材料覆在表面,以增加种植体表面生物活性的处理。用于氧化锆种植体的涂层材料主要有羟基磷灰石(Hydroxyapatite,HA)和生物玻璃陶瓷,如生物玻璃BIOGLASS及CaP类材料等。自Hench[29]发明了生物玻璃(BIOGLASS)以来,生物玻璃陶瓷材料因具有极高的生物相容性而广受关注,并成为近年来的研究热点。除了BIOGLASS,还有磷酸三钙陶瓷(TCP),羟基磷灰石和磷酸钙骨水泥(CPC)等生物玻璃陶瓷材料的相继出现,它们的最终成分为羟基磷灰石,其具有与骨组织相似的结构和成分,具有骨诱导能力,使种植体与骨形成化学结合,能够缩短骨结合的时间和减缓种植后种植体边缘骨吸收的速度,提高了初期骨结合强度,被应用于处理种植体表面形成涂层,即表面涂层处理。 表面涂层处理存在的最大问题是在种植体长期的应用中,涂层存在逐渐与种植体分离的倾向。为了解决这个问题,学者们做了大量的材料改良研究。虽然BIOGLASS在体内能几天内与骨组织形成化学结合[29],但由于它的高结晶倾向和较高的膨胀系数(CTE[BIOGLASS]=15•10–6 K–1,CTE[氧化锆]=10.8- 12.5•10–6 K–1),它不适用于直接涂覆在氧化锆种植体材料表面。研究发现,BIOGLASS材料中的Na2O用K2O代替、CaO用MgO代替可以降低硅基玻璃的热膨胀系数和它的结晶倾向。Kirsten等[30]将BIOGLASS材料中的Na2O用K2O代替、CaO用MgO代替形成的新型玻璃材料,发现其热膨胀系数(11.58•10–6 K–1)略低于氧化锆的(11.67•10–6 K–1),且能与氧化锆材料形成强结合力,在体外的模拟体液中表现出了良好的生物活性。 Kim等[31]发现磷酸钙盐与磷酸盐基玻璃的复合材料涂层的附着力高达40 MPa,远高于纯HA涂层的附着力(22 MPa),且其具有与羟基磷灰石相似的良好的细胞相容性。Oliva等[32]评价了831个氧化锆种植体5年成功率,发现生物陶瓷(Na2O-K2O-MgO-Al2O3-CaO-SiO2-P2O5-F)涂层烧结+酸蚀处理的氧化锆种植体的5年成功率(97.60%)显著性高于该生物陶瓷涂层烧结处理的(93.57%)和机械加工处理的(92.77%)。Oliva认为这种差异可能是表面粗糙度的不同造成的,前者的粗糙度Sa为1.16 μm,后两者的粗糙度Sa分别为0.92和0.62 μm。 2.2.2 商品化的氧化锆种植体系统 现今,以往研究中主要的商品化氧化锆种植体系统共有20个品牌,如表1所示。其中,最常见的表面处理技术是喷砂、酸蚀和喷砂酸蚀。最早的氧化锆种植体以一段式结构出现,其留存率为74%-98%[33]。随着研究的进展,特别是连接加工技术的改进,出现了两段式的氧化锆种植体。两段式结构更灵活,可避免愈合期内有害的负重,更利于种植体的早期稳定和骨结合,其4年成功率为96.7%-99.4%。"
| [1]Adell R, Lekholm U, Rockler B, et al. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10(6):387-416.[2]Heydecke G, Kohal R, Glaser R. Optimal esthetics in single-tooth replacement with the Re-Implant system: a case report. Int J Prosthodont. 1999;12(2):184-189.[3]Weingart D, Steinemann S, Schilli W, et al. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int J Oral Maxillofac Surg. 1994;23(6 Pt 2):450-452.[4]程宁,施斌.氧化锆种植体的研究进展[J].口腔医学研究, 2011, 27(12):1115-1117.[5]Stadlinger B, Hennig M, Eckelt U, et al. Comparison of zirconia and titanium implants after a short healing period. A pilot study in minipigs. Int J Oral Maxillofac Surg. 2010;39(6): 585-592.[6]Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004;17(5):536-543.[7]Mueller C K, Solcher P, Peisker A, et al. Analysis of the influence of the macro- and microstructure of dental zirconium implants on osseointegration: a minipig study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e1-e8.[8]Gahlert M, Gudehus T, Eichhorn S, et al. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin Oral Implants Res. 2007;18(5): 662-668.[9]Chintapalli RK, Marro FG, Jimenez-Pique E, et al. Phase transformation and subsurface damage in 3Y-TZP after sandblasting. Dent Mater. 2013;29(5):566-572.[10]Brull F, Van Winkelhoff AJ, Cune MS. Zirconia dental implants: a clinical, radiographic, and microbiologic evaluation up to 3 years. Int J Oral Maxillofac Implants. 2014;29(4):914-920.[11]戴文雍,印厚才,陈晨,等.热酸溶液蚀刻对氧化锆粘接强度的影响[J].华西口腔医学杂志,2013,31(6):569-573.[12]Flamant Q, Marro FG, Rovira JJR, et al. Hydrofluoric acid etching of dental zirconia. Part 1: etching mechanism and surface characterization. J Eur Ceram Soc. 2016;36(1): 121-134. [13]Gahlert M, Roehling S, Sprecher CM, et al. In vivo performance of zirconia and titanium implants: a histomorphometric study in mini pig maxillae. Clin Oral Implants Res. 2012;23(3):281-286.[14]Zhao G, Raines AL, Wieland M, et al. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007;28(18):2821-2829.[15]Hirano T, Sasaki H, Honma S, et al. Proliferation and osteogenic differentiation of human mesenchymal stem cells on zirconia and titanium with different surface topography. Dent Mater J. 2015;34(6):872-880.[16]Bergemann C, Duske K, Nebe J B, et al. Microstructured zirconia surfaces modulate osteogenic marker genes in human primary osteoblasts. J Mater Sci Mater Med. 2015; 26(1): 5350.[17]Schliephake H, Hefti T, Schlottig F, et al. Mechanical anchorage and peri-implant bone formation of surface-modified zirconia in minipigs. J Clin Periodontol. 2010;37(9):818-828.[18]Aboushelib MN, Salem NA, Taleb AL, et al. Influence of surface nano-roughness on osseointegration of zirconia implants in rabbit femur heads using selective infiltration etching technique. J Oral Implantol. 2013;39(5):583-590.[19]Hao L, Lawrence J, Chian K S. Osteoblast cell adhesion on a laser modified zirconia based bioceramic. J Mater Science Mater Med. 2005;16(8):719-726.[20]Hauser-Gerspach I, Stubinger S, Meyer J. Bactericidal effects of different laser systems on bacteria adhered to dental implant surfaces: an in vitro study comparing zirconia with titanium. Clin Oral Implants Res. 2010;21(3):277-283.[21]Kae Kakura KY, Taniguchi Y, Yamamoto Y, et al. Zirconia Implant with Rough Surface Produced by YAG Laser Treatment Evaluation of Histomorphology and Strength of Osseointegration. J Hard Tissue Biol. 2013;23(1):77-82.[22]Kimie Yasuno KK, Taniguchi Y, Yamaguchi Y, et al. Zirconia Implants with Laser Surface Treatment Peri-Implant Bone Response and Enhancement of Osseointegration. J Hard Tissue Biol. 2014;23(1):93-100.[23]Taniguchi Y, Kakura K, Yamamoto K, et al. Accelerated osteogenic differentiation and bone formation on zirconia with surface grooves created with fiber laser irradiation. Clin Implant Dent Relat Res. 2015.[24]Calvo-Guirado JL, Aguilar-Salvatierra A, Gomez-Moreno G, et al. Histological, radiological and histomorphometric evaluation of immediate vs. non-immediate loading of a zirconia implant with surface treatment in a dog model. Clin Oral Implants Res. 2014;25(7):826-830.[25]Noro A, Kaneko M, Murata I, et al. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal). J Biomed Mater Res B Appl Biomater. 2013;101(2):355-363.[26]Vogler EA. Structure and reactivity of water at biomaterial surfaces. Adv Colloid Interface Sci. 1998;74:69-117.[27]Yang Y, Zhou J, Liu X, et al. Ultraviolet light-treated zirconia with different roughness affects function of human gingival fibroblasts in vitro: the potential surface modification developed from implant to abutment. J Biomed Mater Res B Appl Biomater. 2015;103(1):116-124.[28]Tuna T, Wein M, Altmann B, et al. Effect of ultraviolet photofunctionalisation on the cell attractiveness of zirconia implant materials. Eur Cells Mater. 2015;29:82-96.[29]Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006;17(11):967-978.[30]Kirsten A, Hausmann A, Weber M, et al. Bioactive and thermally compatible glass coating on zirconia dental implants. J Dent Res. 2015;94(2):297-303.[31]Kim HW, Georgiou G, Knowles JC, et al. Calcium phosphates and glass composite coatings on zirconia for enhanced biocompatibility. Biomaterials. 2004;25(18):4203-4213.[32]Oliva J, Oliva X, Oliva JD. Five-year success rate of 831 consecutively placed Zirconia dental implants in humans: a comparison of three different rough surfaces. Int J Oral Maxillofac Implants. 2010;25(2):336-344.[33]Jank S, Hochgatterer G. Success rate of two-piece zirconia implants: a retrospective statistical analysis. Implant Dent. 2016;25(2):193-198. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Zhang Jianguo, Chen Chen, Hu Fengling, Huang Daoyu, Song Liang. Design and biomechanical properties of dental implant pore structure based on three-dimensional finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 585-590. |
| [7] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [8] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [9] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [10] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [11] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [12] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [13] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||