Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (10): 1520-1526.doi: 10.3969/j.issn.2095-4344.2017.10.008
Previous Articles Next Articles
The slow release performance of calcium sulfate/poly(amino acid) compound materials carrying triple anti-tuberculosis drugs in a rabbit model of spinal tuberculosis
Wang Qian1, Geng Guang-qi2, Cong Xiao-ming3, Liu Hai-tao2, Shi Jian-dang2, Wang Zi-li2, Ma Wen-xin2, Sun Yu-hang4
- 1 School of Pharmacy, University of South Florida, Tampa 33612, USA; 2 Department of Spine, General Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; 3 Department of Orthopedics, Weihaiwei People’s Hospital, Weihai 264200, Shandong Province, China; 4 Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China
-
Received:2017-02-12Online:2017-04-08Published:2017-05-08 -
Contact:Shi Jian-dang, Department of Spine, General Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China -
About author:Wang Qian, Master, Attending physician, School of Pharmacy, University of South Florida, Tampa 33612, USA -
Supported by:the National Natural Science Foundation of China, No. 81060149; the Natural Science Foundation of Ningxia Hui Autonomous Region, No. NZ10117
CLC Number:
Cite this article
Wang Qian1, Geng Guang-qi2, Cong Xiao-ming3, Liu Hai-tao2, Shi Jian-dang2, Wang Zi-li2, Ma Wen-xin2, Sun Yu-hang4 . The slow release performance of calcium sulfate/poly(amino acid) compound materials carrying triple anti-tuberculosis drugs in a rabbit model of spinal tuberculosis[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(10): 1520-1526.
share this article
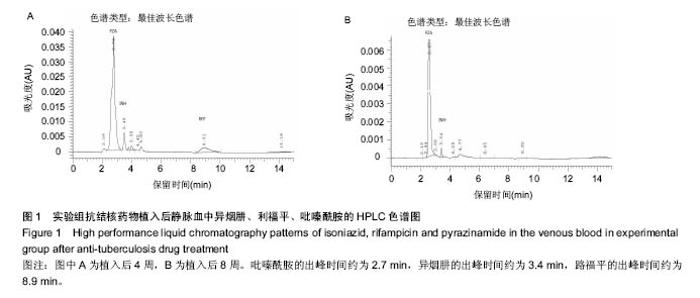
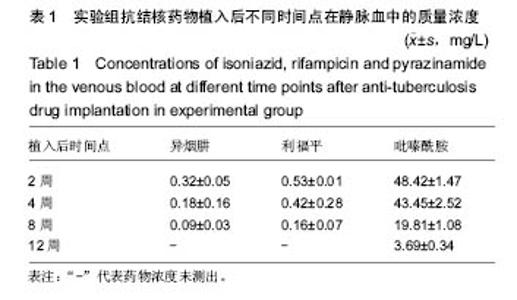
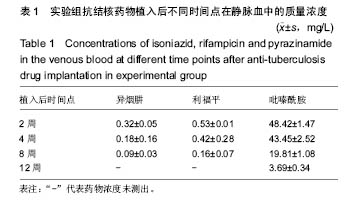
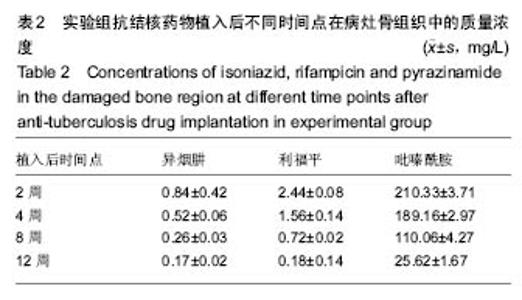
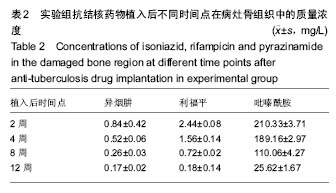
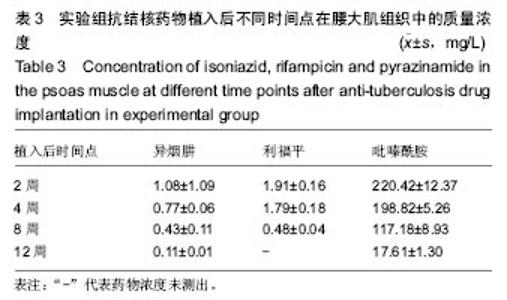
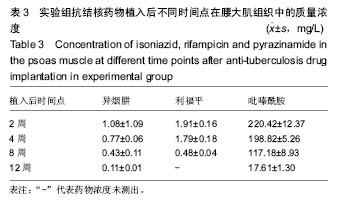
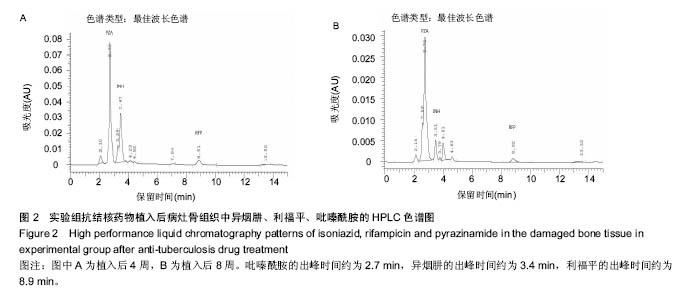
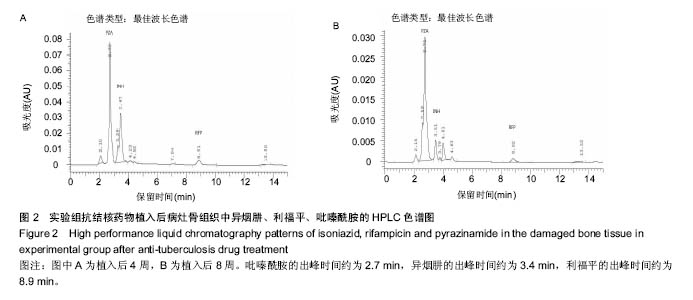
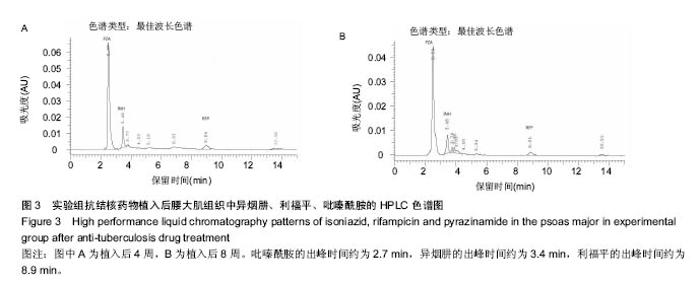
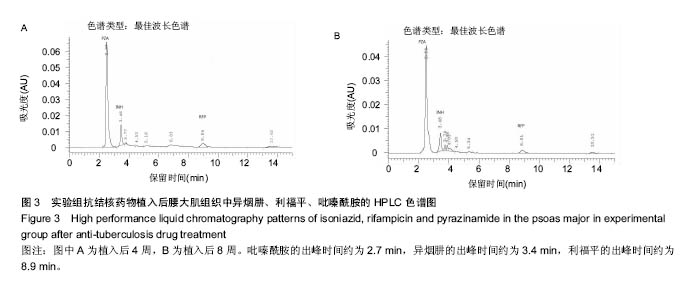
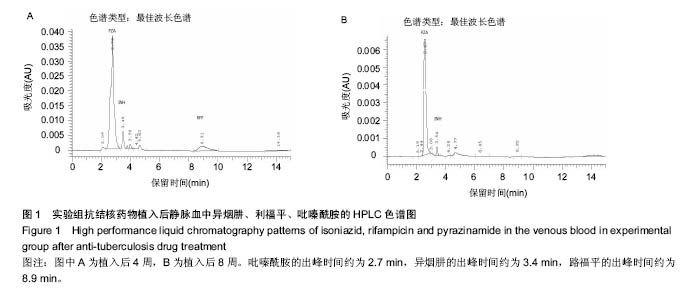
由实验组HPLC色谱图可知,吡嗪酰胺的出峰时间约为2.7 min,异烟肼的出峰时间约为3.4 min,利福平的出峰时间约为8.9 min (图1-3)。对照组未检测到抗痨药物浓度,测得色谱图用于基线校对。 文献表明,异烟肼对结核杆菌的最低抑菌浓度为 0.02-0.05 mg/L,最低杀菌浓度为10倍的最低抑菌浓度[18-19]; 利福平对结核分枝杆菌和其他分枝杆菌的最低抑菌浓度为0.39-1.56 mg/L、最低杀菌浓度为0.78-3.125 mg/L;吡嗪酰胺的最低抑菌浓度还存在争议,当在pH=5.5的环境中的最低抑菌浓度为12.5 mg/L,在50 mg/L时即有杀灭结核分枝杆菌的功效。 异烟肼植入后2周时,在静脉血、病灶骨组织及腰大肌中均达到最低杀菌浓度;植入后4,8周时,在病灶骨组织及腰大肌中达到最低杀菌浓度,在静脉血中仅达到最低抑菌浓度;植入后12周时,病灶骨组织及腰大肌中达到最低抑菌浓度,而静脉血中未测到。 植入后2,4周时,利福平在病灶骨组织及腰大肌中均可达到最低杀菌浓度,但在静脉血中仅仅达到最低抑菌浓度的水平;植入后8周时,在病灶骨组织及腰大肌中仍然可达到最低抑菌浓度,而此时静脉血中未达到最低抑菌浓度;植入后12周时,在病灶骨组织中可检测到存在,但未达到最低抑菌浓度水平,而腰大肌及静脉血中未测到利福平。 植入后2,4,8周时,吡嗪酰胺在病灶骨组织及腰大肌中均达到最低杀菌浓度水平,同时在静脉血中也均达到最低抑菌浓度水平;植入后12周时,在病灶骨组织及腰大肌中仍然能够达到最低抑菌浓度水平,此时在静脉血中吡嗪酰胺未达到最低抑菌浓度水平。 使用Kruskal-Wallis H 检验,当P < 0.01时,按照a=0.05的水准,则认为同一时间两个样本药物浓度存在统计学意义。然后用Nemenyi法进行两两样本之间的比较,采用a=0.05的水准,认为同一时间腰大肌与病灶骨组织中药物浓度没有差别,而同一时间病灶骨组织中的药物浓度高于同期静脉血中的药物浓度,同时同一时间腰大肌中药物浓度则要高于同期静脉血中的药物浓度。 "
| [1]戈朝晖,王自立,魏敏吉,等.脊柱结核病灶中抗痨药物浓度的测定[J].中华骨科杂志,2005,25(2):97-101.[2]戈朝晖,王自立,魏敏吉.利福平在脊柱结核患者不同组织分布的实验研究[J].中国脊柱脊髓杂志,2004,14(12):33-36.[3]Wang Z,Ge Z,Jin W,et al.Treatment of spinal tuberculosis with ultrashort- course chemotherapy in conjunction with partial excision of pathologic vertebrae.Spine J.2007;7(6):671-681.[4]Diacon AH,Donald PR.The early bactericidal activity of antituberculosis drugs.Expert Rev Anti Infect Ther.2014;12(2): 223-237. [5]Vora C,Patadia R,Mittal K,et al.Recent patents and advances on anti-tuberculosis drug delivery and formulations.Recent Pat Drug Deliv Formul.2013;7(2):138-149.[6]罗翼,邹昌,张帅,等.注射型可吸收氨基酸聚合物/硫酸钙复合材料动物体内降解吸收及促成骨作用实验研究[J].华西医学, 2015,20(12):2224-2228.[7]Turner TM,Urban RM,Gitelis S,et al.Resorption evaluation of a large bolus of calcium sulfate in a canine medullary defect.Orthopedics.2003;26(5 Suppl):s577-579.[8]赵增辉,蒋电明,苏保,等.复合骨修复材料——聚氨基酸/硫酸钙的生物相容性研究[J].功能材料,2011,5(42):807-811.[9]德向研,施建党,王自立,等.载三联抗结核药硫酸钙/氨基酸聚合物人工骨体内缓释实验研究[J].中国脊柱脊髓杂志,2013, 23(6): 531-536.[10]刘海涛,施建党,王骞,等.硫酸钙/聚氨基酸复合三联抗痨药人工缓释材料的制备及物理性能测定[J].中国矫形外科杂志,2015, 23(21):1984-1988.[11]刘海涛,王自立,施建党.人工骨载三联抗痨药物含量对其抗机械压缩强度的影响[J].宁夏医科大学学报,2013,35(7):759-762.[12]刘海涛,施建党,王骞,等.载三联抗结核药物硫酸钙/聚氨基酸人工材料体外缓释性能的观察[J].中国脊柱脊髓杂志,2015,25(3): 239-244.[13]张峻山,王自立,施建党,等.复合三联抗结核药人工缓释材料体外抗结核性能[J].脊柱外科杂志,2014,12(6):380-384.[14]耿广起,王骞,王自立,等.构建新西兰兔脊柱结核模型的对比研究[J].中华骨科杂志,2014,34(2):216-223.[15]Geng G,Wang Q,Shi J,et al.Establishment of a new zealand rabbit model of spinal tuberculosis.J Spinal Disord Tech.2015; 28(3):E140-145.[16]曹烨,宋言峥,李垒,等.经典病灶清除手术治疗复杂复合性脊柱结核104例临床分析[J].中国抗痨杂志,2012,34(7):437-440. [17]Song SH,Jun SH,Park KU,et al.Simultaneous determination of first-line anti-tuberculosis drugs and their major metabolic ratios by liquid chromatography/tandem mass spectr ometry.Rapid Commun Mass Spectrum.2008;21(7):1331-1338.[18]张立群,李华,巩维进.结核病的化学治疗[J].中国临床医生,2009, 37(21):165-166.[19]周林.抗结核药物研究进展[J].中国临床医生,2013,41(3):14-17.[20]王自立.病灶清除单节段融合固定治疗脊柱结核[J].中国脊柱脊髓杂志,2009,19(11):807.[21]Khuhawar MY,Rind FM.Liquid chromatographic determination of isoniazid, pyrazinamide and rifampicin from pharmaceutical preparations and blood.J Chromatogr B Analyt Technol Biomed Life Sci.2002;766(2):357-363.[22]李群力,蒋晓萌,施存元.高效液相色谱法测定复方利福平片中利福平、异烟肼及吡嗪酰胺的含量[J].中国抗生素杂志, 2002, 27(9):570-571.[23]Buchholz HW,Engelbrecht H.Depot effects of various antibiotics mixed with Palacos resins.Chirurg. 1970;41(11): 511-515.[24]Pang QY,Lu Y,Yu L,et al.Analytical separation of rifampicin, isoniazid and pyrazinamide in compound rifampicin tablets by RP-HPLC.Chin J Pharma Anal.1998;18(4):259.[25]胡咏梅,徐新华,徐建明.HPLC法测定血清中异烟肼、利福平和吡嗪酰胺浓度[J].安徽医科大学学报,2002,37(5):372-374.[26]金卫东,王骞,王自立,等.彻底与非彻底病灶清除术治疗脊柱结核的比较[J].中华骨科杂志,2014,34(2):196-203.[27]Guo H,Wei J,Liu CS.Development of a degradable cement of calcium phosphate and calcium sulfate composite for bone reconstruction.Biomed Mater.2006;1(4):193-197.[28]Urban RM,Turner TM,Hall DJ,et al.Increased bone formation using calcium sulfate-calcium phosphate composite graft.Clin Orthop Relat Res.2008;459:110-117.[29]Civinini R,De B,Carulli C,et al.The use of an injectable calcium sulphate/calcium phosphate bioceramic in the treatment of osteonecrosis of the femoral head.Int Orthop. 2012; 36(8):1583-1588.[30]Liu P,Jiang H,Li S,et al.Determination of anti-tuberculosis drug concentration and distribution from sustained release microspheres in the vertebrae of a spinal tuberculosis rabbit model.J Orthop Res.2017;35(1):200-208. [31]Dong J,Zhang S,Liu H,et al.Novel alternative therapy for spinal tuberculosis during surgery: reconstructing with anti-tuberculosis bioactivity implants.Expert Opin Drug Deliv.2014;11(3):299-305. [32]Xi YH,Xue MT,Ye XJ,et al.Osteogenetic capacity of cross-linked adjustable anti-tuberculosis drug sustained-release artificial composite.Zhonghua Yi Xue Za Zhi.2013;93(19):1494-1498. [33]Wang P,Liu P,Peng H,et al.Biocompatibility evaluation of dicalcium phosphate/calcium sulfate/poly (amino acid) composite for orthopedic tissue engineering in vitro and in vivo.J Biomater Sci Polym Ed. 2016;27(11):1170-1186.[34]Xue Y,Song Y,Liu L,et al.Experimental study on poly-amino acid/nano-hydroxyapatite/calcium sulfate cage for lumbar intervody fusion in goats.Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.2015;29(8):972-977. [35]Fan X,Ren H,Luo X,et al.Mechanics, degradability, bioactivity, in vitro, and in vivo biocompatibility evaluation of poly(amino acid)/hydroxyapatite/calcium sulfate composite for potential load-bearing bone repair.J Biomater Appl.2016;30(8): 1261-1272. [36]Mine Y,Zhang H.Anti-inflammatory Effects of Poly-L-lysine in Intestinal Mucosal System Mediated by Calcium-Sensing Receptor Activation.J Agric Food Chem.2015;63(48): 10437-10447. [37]Nayunigari MK,Gupta SK,Kokkarachedu V,et al.Development of anti-scale poly(aspartic acid-citric acid) dual polymer systems for water treatment.Environ Technol. 2014;35(21-24): 2903-2909. [38]Tan V,Evaniew N,Finlay K,et al.Chronology of the Radiographic Appearances of the Calcium Sulfate-Calcium Phosphate Synthetic Bone Graft Composite Following Resection of Bone Tumors: A Follow-up Study of Postoperative Appearances.Can Assoc Radiol J. 2016;67(1): 21-27. [39]Evaniew N,Tan V,Parasu N,et al.Use of a calcium sulfate-calcium phosphate synthetic bone graft composite in the surgical management of primary bone tumors. Orthopedics. 2013;36(2):e216-222. [40]Mistry S,Roy S,Maitra NJ,et al.A novel, multi-barrier, drug eluting calcium sulfate/biphasic calcium phosphate biodegradable composite bone cement for treatment of experimental MRSA osteomyelitis in rabbit model.J Control Release. 2016;239:169-181.[41]Wu CC,Hsu LH,Tsai YF,et al.Enhancement of biodegradation and osseointegration of poly(ε-caprolactone)/calcium phosphate ceramic composite screws for osteofixation using calcium sulfate.Biomed Mater.2016;11(2):025012. [42]Zhou Z,Buchanan F,Mitchell C,et al.Printability of calcium phosphate: calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique.Mater Sci Eng C Mater Biol Appl.2014;38:1-10. [43]Al Khudairy A,Phelan JP.Letter to the editor: in vitro elution characteristics of vancomycin in a composite calcium phosphate/ calcium sulfate bone substitute.HSS J. 2014;10(1):98. [44]Yang X,Osagie L,Bostrom MP.In vitro elution characteristics of vancomycin in a composite calcium phosphate/calcium sulfate bone substitute.HSS J.2012;8(2):129-32. [45]Cai S,Zhai Y,Xu G,et al.Preparation and properties of calcium phosphate cements incorporated gelatin microspheres and calcium sulfate dihydrate as controlled local drug delivery system.J Mater Sci Mater Med.2011;22(11):2487-2496. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Chen Jinmin, Chen Suisheng, Ding Jing, Xia Baoquan, Luo Xiaojia, Lu Chenghai. Stability of balloon dilation with injectable calcium sulfate cement for tibial plateau fractures [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 414-418. |
| [13] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [14] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [15] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||