Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (9): 1390-1396.doi: 10.3969/j.issn.2095-4344.2017.09.015
Previous Articles Next Articles
The overexpression of miR-378 promotes the therapeutic effects of bone marrow mesenchymal stem cell transplantation on myocardial infarction
Zhang Xiu-min1, 2, Yu Bo1, Li Xue-yuan1
- 1Department of Circulatory Medicine, the First Hospital of China Medical University, Shenyang 110003, Liaoning Province, China; 2Department of Circulatory Medicine, General Hospital of Fushun Mining Bureau, Fushun 113008, Liaoning Province, China
-
Online:2017-03-28Published:2017-03-31 -
Contact:Yu Bo, M.D., Professor, Chief physician, Department of Circulatory Medicine, the First Hospital of China Medical University, Shenyang 110003, Liaoning Province, China -
About author:Zhang Xiu-min, Associate chief physician, Department of Circulatory Medicine, the First Hospital of China Medical University, Shenyang 110003, Liaoning Province, China; Department of Circulatory Medicine, General Hospital of Fushun Mining Bureau, Fushun 113008, Liaoning Province, China -
Supported by:the Science and Technology Plan of Liaoning Province, No. 2012225084; the Science and Technology Plan of Shenyang, No. F12-193-9-03, F16-206-9-08; the Scientific Research Project of Liaoning Provincial Department of Education, No. L2013316
CLC Number:
Cite this article
Zhang Xiu-min, Yu Bo, Li Xue-yuan. The overexpression of miR-378 promotes the therapeutic effects of bone marrow mesenchymal stem cell transplantation on myocardial infarction[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(9): 1390-1396.
share this article
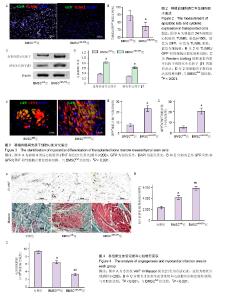
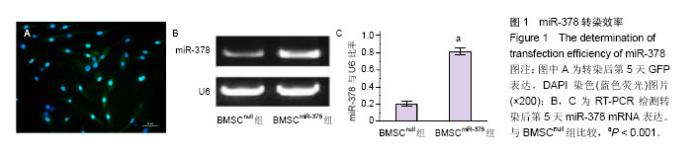
2.2 移植后细胞凋亡率及细胞因子表达 移植24 h后,TUNEL染色结果显示(图2A,B)BMSCnull组移植骨髓间充质干细胞凋亡比率为(88.5±6.7)%,而BMSCmiR-378组的细胞凋亡比率明显减低[(74.9±5.4)%,n=6,P < 0.001],提示miR-378转染明显抑制移植骨髓间充质干细胞的凋亡。Western blotting结果显示(图2C和D),BMSCmiR-378组血管内皮生长因子和转化生长因子β1的蛋白表达水平明显高于BMSCnull组(n=6,P < 0.001),提示miR-378转染明显提高移植骨髓间充质干细胞的旁分泌功能。 2.3 移植骨髓间充质干细胞存活与心肌细胞分化 移植4周后,免疫荧光染色结果显示(图3A-C),BMSCmiR-378组中GFP+细胞数量明显高于BMSCnull组[(23.4±3.1)/高倍视野,(7.6±1.7)/高倍视野,n=10,P < 0.001],提示miR-378转染增加骨髓间充质干细胞存活数量。另外,BMSCmiR-378组中GFP+/cTNT+细胞数量亦明显高于BMSCnull组[(20.6±2.9)/高倍视野,(5.2±1.3)/高倍视野,n=10,P < 0.001],提示miR-378转染促进骨髓间充质干细胞向心肌细胞分化。 2.4 血管再生与心肌梗死面积评价 移植4周后,抗vWF染色结果显示(图4A,B)BMSCmiR-378组新生血管密度明显高于BMSCnull组和对照组[(5 839±353)像素/高倍视野, (4 148±296)像素/高倍视野,(2 314±262)像素/高倍视野,n=10,P均< 0.001]。Masson染色提示(图4A,C),BMSCmiR-378组心肌梗死面积明显小于BMSCnull组和对照组[(33 716±2 851)像素/高倍视野,(64 288±3 590)像素/高倍视野,(91 626±3 817)像素/高倍视野,n=10,P < 0.001]。与对照组比较,BMSCnull组新生血管密度和心肌梗死面积差异也有显著性意义(P < 0.001)。 2.5 心功能与心室重构 术后4周,BMSCmiR-378组左室射血分数[(58±3)%]明显优于BMSCnull组[(50±3)%,n=10,P=0.016]和对照组[(41±2)%,n=10,P < 0.001];左室舒张末容积[(1.82±0.16) mL]较BMSCnull组[(2.21± 0.19) mL,n=10,P=0.037]和对照组[(2.64±0.22) mL,n=10,P=0.003]亦有明显改善。BMSCnull组与对照组比较也有改善(P < 0.05),证实了未处理骨髓间充质干细胞的治疗效果。"

| [1] Petrie MC, Jhund PS, She L, et al. Ten-Year Outcomes After Coronary Artery Bypass Grafting According to Age in Patients With Heart Failure and Left Ventricular Systolic Dysfunction: An Analysis of the Extended Follow-Up of the STICH Trial (Surgical Treatment for Ischemic Heart Failure). Circulation. 2016 1;134(18):1314-1324.[2] de Waha A, Sandner S, von Scheidt M, et al. A randomized, parallel group, double-blind study of ticagrelor compared with aspirin for prevention of vascular events in patients undergoing coronary artery bypass graft operation: Rationale and design of the Ticagrelor in CABG (TiCAB) trial: An Investigator-Initiated trial. Am Heart J. 2016 p; 179: 69-76.[3] Lam CS, Teng TK, Tay WT, et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J. 2016;37(41):3141-3153.[4] Bertrand ME, Ferrari R, Remme WJ, et al. Perindopril and β-blocker for the prevention of cardiac events and mortality in stable coronary artery disease patients: A EUropean trial on Reduction Of cardiac events with Perindopril in stable coronary Artery disease (EUROPA) subanalysis. Am Heart J. 2015;170(6):1092-1098.[5] Reich H, Tseliou E, de Couto G, et al. Repeated transplantation of allogeneic cardiosphere-derived cells boosts therapeutic benefits without immune sensitization in a rat model of myocardial infarction. J Heart Lung Transplant. 2016;35(11):1348-1357.[6] Gautam M, Fujita D, Kimura K, et al. Transplantation of adipose tissue-derived stem cells improves cardiac contractile function and electrical stability in a rat myocardial infarction model. J Mol Cell Cardiol. 2015;81:139-149.[7] Shudo Y, Miyagawa S, Ohkura H, et al. Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model. Tissue Eng Part A. 2014b;20(3-4): 728-739.[8] Zhang GW, Gu TX, Guan XY, et al. HGF and IGF-1 promote protective effects of allogeneic BMSC transplantation in rabbit model of acute myocardial infarction. Cell Prolif. 2015;48(6): 661-670.[9] Yu J, Li M, Qu Z, et al. SDF-1/CXCR4-mediated migration of transplanted bone marrow stromal cells toward areas of heart myocardial infarction through activation of PI3K/Akt. J Cardiovasc Pharmacol. 2010;55(5):496-505.[10] Peng C, Pei H, Wei F, et al. Cellular repressor of E1A-stimulated gene overexpression in bone mesenchymal stem cells protects against rat myocardial infarction. Int J Cardiol. 2015;183:232-241.[11] Russo V, Young S, Hamilton A, et al. Mesenchymal stem cell delivery strategies to promote cardiac regeneration following ischemic injury. Biomaterials. 2014;35(13):3956-3974.[12] Hua P, Wang YY, Liu LB, et al. In vivo magnetic resonance imaging tracking of transplanted superparamagnetic iron oxide-labeled bone marrow mesenchymal stem cells in rats with myocardial infarction. Mol Med Rep. 2015;11(1):113-120.[13] Jin P, Wang E, Wang YH, et al. Central zone of myocardial infarction: a neglected target area for heart cell therapy. J Cell Mol Med. 2012;16(3):637-648.[14] Liu XB, Wang JA, Ji XY, et al. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Res Ther. 2014;5(5):111.[15] Fang J, Chen L, Fan L, et al. Enhanced therapeutic effects of mesenchymal stem cells on myocardial infarction by ischemic postconditioning through paracrine mechanisms in rats. J Mol Cell Cardiol. 2011;51(5):839-847.[16] Zhong Z, Hu JQ, Wu XD, et al. Myocardin-related transcription factor-A-overexpressing bone marrow stem cells protect cardiomyocytes and alleviate cardiac damage in a rat model of acute myocardial infarction. Int J Mol Med. 2015; 36(3):753-759.[17] Chacko SM, Khan M, Kuppusamy ML, et al. Myocardial oxygenation and functional recovery in infarct rat hearts transplanted with mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2009;296(5):H1263-273.[18] Zhu C, Yu J, Pan Q, et al. Hypoxia-inducible factor-2 alpha promotes the proliferation of human placenta-derived mesenchymal stem cells through the MAPK/ERK signaling pathway. Sci Rep. 2016;6:35489.[19] Saini U, Gumina RJ, Wolfe B, et al. Preconditioning mesenchymal stem cells with caspase inhibition and hyperoxia prior to hypoxia exposure increases cell proliferation. J Cell Biochem. 2013;114(11):2612-2623.[20] Lee DY, Deng Z, Wang CH, et al. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104(51):20350-20355.[21] Yu BL, Peng XH, Zhao FP, et al. MicroRNA-378 functions as an onco-miR in nasopharyngeal carcinoma by repressing TOB2 expression. Int J Oncol. 2014;44(4):1215-1222.[22] Matkovich SJ, Hu Y, Dorn GW 2nd. Regulation of cardiac microRNAs by cardiac microRNAs. Circ Res. 20131;113(1): 62-71.[23] Naga Prasad SV, Duan ZH, Gupta MK, et al. Unique microRNA profile in end-stage heart failure indicates alterations in specific cardiovascular signaling networks. J Biol Chem. 2009;284(40):27487-27499.[24] Xing Y, Hou J, Guo T, et al. microRNA-378 promotes mesenchymal stem cell survival and vascularization under hypoxic-ischemic conditions in vitro. Stem Cell Res Ther. 2014;5(6):130.[25] Zhang N, Zhong J, Han S, et al. MicroRNA-378 Alleviates Cerebral Ischemic Injury by Negatively Regulating Apoptosis Executioner Caspase-3. Int J Mol Sci. 2016;17(9): E1427.[26] Fang J, Song XW, Tian J, et al. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis. 2012;17(4):410-423.[27] Ding R, Jiang X, Ha Y, et al. Activation of Notch1 signalling promotes multi-lineage differentiation of c-Kit(POS)/ NKX2.5(POS) bone marrow stem cells: implication in stem cell translational medicine. Stem Cell Res Ther. 2015;6:91.[28] Lu DF, Wang Y, Su ZZ, et al. Knockdown of the HDAC1 promotes the directed differentiation of bone mesenchymal stem cells into cardiomyocytes. PLoS One. 2014;9(3): e92179.[29] Li P, Zhang L. Exogenous Nkx2.5- or GATA-4-transfected rabbit bone marrow mesenchymal stem cells and myocardial cell co-culture on the treatment of myocardial infarction in rabbits. Mol Med Rep. 2015g;12(2):2607-2621.[30] Ju H, Yang Y, Sheng A, et al. MicroRNA-378 promotes myogenic differentiation by targeting BMP4. Mol Med Rep. 2016;13(3):2194-2200.[31] Del Ry S, Cabiati M, Martino A, et al. High concentration of C-type natriuretic peptide promotes VEGF-dependent vasculogenesis in the remodeled region of infarcted swine heart with preserved left ventricular ejection fraction. Int J Cardiol. 2013;168(3):2426-2434.[32] Ho YS, Tsai WH, Lin FC, et al. Cardioprotective Actions of TGFβRI Inhibition Through Stimulating Autocrine/Paracrine of Survivin and Inhibiting Wnt in Cardiac Progenitors. Stem Cells. 2016;34(2):445-455.[33] Kofidis T, de Bruin JL, Yamane T, et al. Stimulation of paracrine pathways with growth factors enhances embryonic stem cell engraftment and host-specific differentiation in the heart after ischemic myocardial injury. Circulation. 2005;111 (19):2486-2493.[34] Muhl L, Moessinger C, Adzemovic MZ, et al. Expression of vascular endothelial growth factor (VEGF)-B and its receptor (VEGFR1) in murine heart, lung and kidney. Cell Tissue Res. 2016;365(1):51-63.[35] Lui KO, Zangi L, Silva EA, et al. Driving vascular endothelial cell fate of human multipotent Isl1+ heart progenitors with VEGF modified mRNA. Cell Res. 2013;23(10):1172-1186.[36] Lee HJ, Cho HJ, Kwon YW, et al. Phenotypic modulation of human cardiospheres between stemness and paracrine activity, and implications for combined transplantation in cardiovascular regeneration. Biomaterials. 2013;34(38): 9819-9829.[37] Lui KO, Zangi L, Chien KR. Cardiovascular regenerative therapeutics via synthetic paracrine factor modified mRNA. Stem Cell Res. 2014;13(3 Pt B):693-704.[38] Gong H, An S, Sassmann A, et al. PAR1 Scaffolds TGFβRII to Downregulate TGF-β Signaling and Activate ESC Differentiation to Endothelial Cells. Stem Cell Reports. 2016; 7(6):1050-1058.[39] Sheu JJ, Lee FY, Yuen CM, et al. Combined therapy with shock wave and autologous bone marrow-derived mesenchymal stem cells alleviates left ventricular dysfunction and remodeling through inhibiting inflammatory stimuli, oxidative stress & enhancing angiogenesis in a swine myocardial infarction model. Int J Cardiol. 2015;193:69-83.[40] Rahbarghazi R, Nassiri SM, Ahmadi SH, et al. Dynamic induction of pro-angiogenic milieu after transplantation of marrow-derived mesenchymal stem cells in experimental myocardial infarction. Int J Cardiol. 2014;173(3):453-466.[41] Windmolders S, De Boeck A, Koninckx R, et al. Mesenchymal stem cell secreted platelet derived growth factor exerts a pro-migratory effect on resident Cardiac Atrial appendage Stem Cells. J Mol Cell Cardiol. 2014;66:177-188. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | Cui Xing, Sun Xiaoqi, Zheng Wei, Ma Dexin. Huangqin Decoction regulates autophagy to intervene with intestinal acute graft-versus-host disease in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1057-1062. |
| [5] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [6] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [7] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [8] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [9] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [10] | Hu Wei, Xie Xingqi, Tu Guanjun. Exosomes derived from bone marrow mesenchymal stem cells improve the integrity of the blood-spinal cord barrier after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 992-998. |
| [11] | Yin Tingting, Du Dayong, Jiang Zhixin, Liu Yang, Liu Qilin, Li Yuntian. Granulocyte colony-stimulating factors improve myocardial fibrosis in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 730-735. |
| [12] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [13] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [14] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [15] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||