Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (6): 969-974.doi: 10.3969/j.issn.2095-4344.2017.06.026
Previous Articles Next Articles
Research advance in the osteointegration of surface bioactive coating on titanium alloys
- Second Hospital of Jilin University, Changchun 130041, Jilin Province, China
-
Received:2016-12-03Online:2017-02-28Published:2017-03-16 -
Contact:Qin Yan-guo, Associate professor, Chief physician, Master’s supervisor, Second Hospital of Jilin University, Changchun 130041, Jilin Province, China -
About author:Liu Guan-cong, Studying for master’s degree, Second Hospital of Jilin University, Changchun 130041, Jilin Province, China. -
Supported by:the Provincial Industry Innovation Project of Jilin Province, No. 2016C037; the Science and Technology Development Program of Jilin Province, No. 20150414006G H
CLC Number:
Cite this article
Liu Guan-cong, Li Rui-yan, Liang Hao-jun, Qin Yan-guo. Research advance in the osteointegration of surface bioactive coating on titanium alloys[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(6): 969-974.
share this article
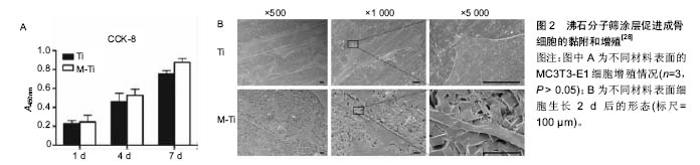
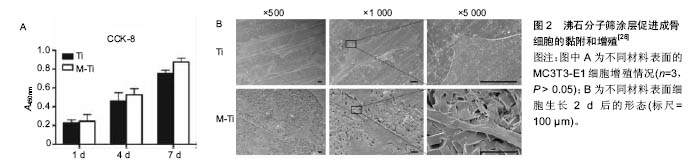
2.1 钛合金表面常用生物活性涂层 2.1.1 磷灰石类涂层 磷灰石是组成人体骨骼的主要无机成分,羟基磷灰石的钙磷比为1.67,磷酸三钙的钙磷比为1.5,均与自然骨的钙磷比(1.71)相近。磷灰石对人体无毒无害,以磷酸三钙及羟基磷灰石为代表的磷酸钙盐矿物是应用最早且最广泛的生物修复材料,但其脆性大、强度低,限制了在负重部位的应用。在钛合金基材表面应用磷灰石类涂层,不仅获得了有效的力学支撑,还发挥了磷灰石的生物活性性能。在植入体表面添加羟基磷灰石涂层被认为是表面改性的标准做法[10],也是评价其他涂层方法是否有效的重要对比标准。羟基磷灰石具有很多优点,但单一的磷灰石涂层与钛合金的热膨胀系数相差较大,涂层制备过程中易产生异常应力而导致结合强度不高[11],易早期脱落。金属基底与涂层之间的残存应力是导致二者结合力不足的重要原因之一,有研究者尝试通过改进制备方法(如等离子喷涂技术)以及在涂层中掺入固溶杂质元素(如锶元素)来达到提高结合力的目的。研究者在单一羟基磷灰石涂层中复合了其他物质,如不同磷灰石类涂层之间的复合,磷灰石类涂层与生物大分子的复合等,不同程度提高了结合强度。Li等[12]通过聚多巴胺辅助羟基磷灰石沉积的方法,在3D打印多孔钛合金表面制备羟基磷灰石涂层,体外实验表明该涂层增强了成骨细胞的黏附、增殖和分化,体内实验同样表明可促进骨整合与骨生成。 磷灰石涂层的生物降解率较高,其自身降解所产生的钙磷离子可进入正常骨代谢,故常用以制备可降解生物涂层,作为药物载体达到长期缓释的效果。Reigstad等[13]以Bonit®(主要成分为透磷钙石)制作生物可吸收涂层,利用兔胫骨和股骨模型研究了磷酸钙类物质吸收时限及骨诱导效果。该研究表明,在植入6周后涂层基本完全吸收,但在植入12周后扭矩实验应力值才有明显提高,这可能与磷灰石涂层促进早期骨细胞黏附、增殖和分化,而骨生成则在稍晚时间发生有关。Cheng等[14]经过研究证明,Ca离子的存在是磷灰石涂层促进骨诱导的关键。 此外,磷灰石类涂层具有多孔结构,涂层的孔隙尺寸、孔隙率是影响骨诱导作用的重要因素。Chan等[15]将不同支架孔隙率(22.5%,32.0%,46.0%)的SiCaP植入绵羊体内,观察到随支架孔隙率的增加,植入物的早期骨诱导作用明显增强。支架孔隙率的增加,可以为植入物周围早期血管生成以及成骨细胞增殖过程中蛋白质的活化提供较大的接触表面积[16],且大孔隙率涂层吸附血清中促成骨分化有关蛋白质的能力更强[17]。Cheng等[18]经过比较大鼠体内不同部位磷酸钙植入物的骨诱导作用,认为磷酸钙生物陶瓷表面从周围内环境中吸附成骨相关生物活性因子(如骨形态发生蛋白2)是其促进骨诱导作用的重要原因。由此可见,多孔羟基磷灰石涂层不仅可以增强成骨细胞长入,还可促进成骨细胞的分化,最终影响成骨效果。 羟基磷灰石可以增强细胞黏附,但羟基磷灰石与钛合金的结合不够牢固,其中最主要的因素是羟基磷灰石的溶解度较大且溶解过于迅速,导致晚期与基底的结合强度下降过快。氟磷灰石是自然界稳定存在的一种物质,其结构实际上是F-替代了羟基磷灰石中的OH-而晶体结构相同。由于F比OH基团小,氟磷灰石比羟基磷灰石晶体结构更加紧密,所以其溶解度较小[19],许多学者尝试在基底上制备氟磷灰石涂层。从化学成分上分析,氟磷灰石中有F离子的溶出,可能对生物体有毒性,但目前未有报道。还有学者认为氟离子不仅可以控制龋齿,还可以刺激骨细胞增殖和分化[20],但其生物活性受涂层厚度、孔隙率等物理性质的影响,对于氟磷灰石是否优于羟基磷灰石仍有较大争议。 2.1.2 生物活性玻璃 生物活性玻璃具有很强的生物活性,尤其是钙钠硅系玻璃(Na2O-CaO-SiO2-P2O5),其结构与玻璃相似,骨诱导能力较强,可最终与骨组织形成稳定的化学键合[21]。与磷灰石相似,生物活性玻璃因强度不足而不具有力学支撑作用,研究者常将其作为涂层应用于钛合金的表面修饰。在制备过程中,可以通过调节各组分所占比例控制涂层的理化性质,使其与钛合金相似或相近,降低二者之间存在的应力差,从而保证了与钛合金基底相结合时的强度。生物活性最强的生物玻璃Bioglass 45S5[21],其各组分的摩尔质量比为24.5% Na2O,24.5% CaO,6% P2O5,45% SiO2,通过调节各组分所占比达到生物活性与结合力的平衡。尽管有关生物活性玻璃的研究开始较早[21],但生物活性玻璃涂层的研究还处于探索阶段,有关其微结构、机械性能的信息较为缺乏[22]。Popa等[23]利用射频磁控溅射技术在钛合金基材表面成功制备了不同化学成分的生物活性玻璃涂层,分析其理化特征与力学、生物学性能之间的关系,表明加入氧有利于增强涂层的结合强度。Cattini等[22]利用等离子喷涂技术在钛合金基材上制备微米级的生物活性涂层,模拟体液浸泡实验显示,该涂层具有良好的生物活性和强度。Cattini研究组之后的研究中,利用等离子喷涂技术制备的生物活性玻璃/羟基磷灰石梯度复合涂层,因不具有残存应力而与基底稳定结合,且生物相容性良好[24]。 此外,有研究者报道生物活性玻璃还具有一定抗炎及增强细胞黏附的作用。Scislowska-Czarnecka等[25]在钛合金表面制备生物活性陶瓷涂层,比较了羟基磷灰石、生物活性玻璃和硅酸钙3种涂层,通过细胞学实验证实生物活性玻璃涂层不仅没有增加炎性反应,还增强了巨噬细胞的黏附能力。相反,硅酸钙涂层却增加了促炎性反应,炎性因子肿瘤坏死因子α,白细胞介素6,白细胞介素12明显增多,同时减弱了巨噬细胞的黏附能力。 2.1.3 新型分子筛涂层 以分子筛为代表的新型生物活性涂层具有规则的微纳结构。分子筛是固体硅酸盐或硅铝酸盐晶体材料,具有规则均匀的微孔结构,又称为沸石(Zeolite)。沸石骨架的基本结构单元为硅氧四面体和铝氧四面体,二者通过共用四面体顶点的氧原子相互连接形成的三维结构具有规则孔道。以沸石分子筛ZSM-5为例,其二维十元环交叉形成两个方向的孔道,其中一个方向为直孔道,另一个方向为“之”字形孔道[26]。特定的孔道形状以及铝氧四面体和硅氧四面体骨架的负电荷,形成沸石分子筛内部的离子交换中心。沸石分子筛的制备过程非常简单,只需要在溶液中浸泡即可在钛合金基底上形成纳米级的分子筛涂层。 分子筛常被用作商业目的的催化、吸附和离子交换。在医学上,沸石也有广泛的应用,不仅可以直接作为抗肿瘤、抗微生物药使用,还可用作药物缓释/靶向载体,MRI造影剂等[26-27]。由此可见,沸石分子筛无细胞毒性,且具有生物活性及生物相容性。 沸石分子筛涂层作为一种新型生物活性涂层,可与Ti6Al4V基底致密的结合。Bedi等[27-29]通过一系列体外实验证明了沸石分子筛涂层可以提高Ti6Al4V基底的生物相容性和耐腐蚀性,还可以促进成骨细胞的黏附和增殖(图2)。Li等[30]在Ti6Al4V表面制备沸石分子筛涂层,首次通过体内实验证明该沸石分子筛涂层具有促进骨生成及改进骨整合的作用。此外,尽管Ti6Al4V有广泛的临床应用,但其在体内释放少量Al和V细胞毒性离子的问题一直没有得到解决。Al和V离子通过影响成骨细胞内成骨相关基因的表达,影响正常的骨愈合过程[31-33]。Bedi等[28]证实,MFI涂层(ZSM-5沸石涂层的简称,是一种具有拓扑结构的分子筛涂层)的高抗腐蚀性不仅可以有效阻止对人体有害的Al和V进入周围组织,还能保护植入物不受人体内环境的腐蚀。 2.2 生物活性涂层的负载作用 多孔羟基磷灰石涂层和分子筛涂层广泛应用,明显延后了翻修手术的时间,但无菌性松动仍是关节置换失败的严重问题[10]。除假体本身材质外,假体周围骨整合起到关键作用,而机体内骨整合过程是多因素相互作用的结果。随着成骨诱导研究的不断深入,研究者注意到了生物活性因子及金属阳性离子在成骨诱导中的重要作用。理想的植入物表面性能应具有2种重要特征[34]:第一是从周围组织中吸引成骨细胞或其祖细胞,并为其提供黏附到植入体表面的锚定点;第二是负载并可以在相对较长时间内持续释放促进成骨细胞增殖分化的离子或细胞因子等物质。常用作载体的涂层主要有羟基磷灰石涂层、生物活性玻璃涂层和分子筛涂层。多孔羟基磷灰石涂层作为生物活性因子或金属离子的载体,起到了长期缓释的效果。此外,羟基磷灰石涂层的降解产物钙、磷等也可以进入正常骨代谢。生物活性玻璃涂层的多孔结构与自然骨相似,可与自然骨形成化学性结合。分子筛孔道内的离子交换中心可吸附各种阳性离子和生物活性因子。 金属阳离子可以改变细胞内DNA转录及蛋白质合成,从而改变细胞的生物活性。大量研究表明,在羟基磷灰石涂层中掺入生物活性离子,如锶[10,35-36]、银[37-38]、锌等[39],可明显提高植入物的骨诱导性及抗菌作用。研究表明,锶离子不仅可以增强成骨细胞活性,还可以抑制破骨细胞活化,从而有效增强骨整合。Wang等[38]在分子筛沸石涂层中加入银离子,有效起到了抗菌作用,减少关节置换后感染的风险。 生物活性因子在骨愈合过程中起重要作用,研究较为深入的骨形态发生蛋白2,可促进骨缺损部位的细胞增殖,并诱导骨髓间充质干细胞向成骨细胞分化。Yoo等[40]利用人工合成的多肽替代骨形态发生蛋白2,同样证明了其在骨愈合早期有促进骨生成的作用。促血管内皮释放因子的引入,可以促进植入物周围血管生成[41]。Ⅰ型胶原蛋白是骨组织中主要的有机物。Xia等[42]将Ⅰ型胶原蛋白复合到羟基磷灰石涂层上,通过细胞实验证实其具有增强早期骨形成的作用。 "
| [1]Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36 Suppl 3:S20-27.[2]Fischer M, Joguet D, Robin G, et al. In situ elaboration of a binary Ti-26Nb alloy by selective laser melting of elemental titanium and niobium mixed powders. Mater Sci Eng C Mater Biol Appl. 2016;62:852-859. [3]Takematsu E, Katsumata K, Okada K, et al. Bioactive surface modification of Ti-29Nb-13Ta-4.6Zr alloy through alkali solution treatments. Mater Sci Eng C Mater Biol Appl. 2016;62:662-667. [4]Tschernitschek H, Borchers L, Geurtsen W. Nonalloyed titanium as a bioinert metal--a review. Quintessence Int. 2005; 36(7-8):523-530.[5]Nine MJ, Choudhury D, Hee AC, et al. Wear Debris Characterization and Corresponding Biological Response: Artificial Hip and Knee Joints. Materials. 2014, 7(2):980-1016.[6]Albrektsson T, Brånemark PI, Hansson HA, et al. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52(2):155-170.[7]戴正宏, 王玉林, 何宝明. 外科植入物用钛合金的表面改性[J]. 稀有金属, 2003, 27(4):491-494.[8]黄伟九, 李兆峰. 医用钛合金表面改性研究进展[J]. 材料导报, 2006, 20(z2):369-372,379.[9]Zhou H, Kong S, Pan Y, et al. Microwave-assisted fabrication of strontium doped apatite coating on Ti6Al4V. Mater Sci Eng C Mater Biol Appl. 2015;56:174-180.[10]Newman SD, Lotfibakhshaiesh N, O'Donnell M, et al. Enhanced osseous implant fixation with strontium-substituted bioactive glass coating. Tissue Eng Part A. 2014;20(13-14):1850-1857.[11]Liu X, Chu PK, Ding C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Materials Science & Engineering R Reports. 2003; 47(3-4):49-121.[12]Li Y, Yang W, Li X, et al. Improving osteointegration and osteogenesis of three-dimensional porous Ti6Al4V scaffolds by polydopamine-assisted biomimetic hydroxyapatite coating. ACS Appl Mater Interfaces. 2015;7(10):5715-5724.[13]Reigstad O, Franke-Stenport V, Johansson CB, et al. Improved bone ingrowth and fixation with a thin calcium phosphate coating intended for complete resorption. J Biomed Mater Res B Appl Biomater. 2007;83(1):9-15.[14]Cheng L, Shi Y, Ye F, et al. Osteoinduction of calcium phosphate biomaterials in small animals. Mater Sci Eng C Mater Biol Appl. 2013;33(3):1254-1260.[15]Chan O, Coathup MJ, Nesbitt A, et al. The effects of microporosity on osteoinduction of calcium phosphate bone graft substitute biomaterials. Acta Biomater. 2012;8(7):2788-2794.[16]Bignon A, Chouteau J, Chevalier J, et al. Effect of micro- and macroporosity of bone substitutes on their mechanical properties and cellular response. J Mater Sci Mater Med. 2003;14(12):1089-1097.[17]El-Ghannam A, Ducheyne P, Shapiro IM. Effect of serum proteins on osteoblast adhesion to surface-modified bioactive glass and hydroxyapatite. J Orthop Res. 1999;17(3):340-345.[18]Cheng L, Ye F, Yang R, et al. Osteoinduction of hydroxyapatite/beta-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater. 2010;6(4):1569-1574.[19]Fahami A, Beall GW, Betancourt T. Synthesis, bioactivity and zeta potential investigations of chlorine and fluorine substituted hydroxyapatite. Mater Sci Eng C Mater Biol Appl. 2016;59:78-85.[20]Bianco A, Cacciotti I, Lombardi M, et al. F-substituted hydroxyapatite nanopowders: Thermal stability, sintering behaviour and mechanical properties. Ceramics International. 2010; 36(1):313-322.[21]Hench LL.The story of Bioglass.J Mater Sci Mater Med. 2006; 17(11):967-978.[22]Cattini A, ?atka L, Bellucci D, et al. Suspension plasma sprayed bioactive glass coatings: Effects of processing on microstructure, mechanical properties and in-vitro behaviour. Surface and Coatings Technology. 2013; 220(5):52-59.[23]Popa AC, Marques VMF, Stan GE, et al. Nanomechanical characterization of bioglass films synthesized by magnetron sputtering. Thin Solid Films. 2014; 553(3):166-172.[24]Cattini A, Bellucci D, Sola A, et al. Microstructural design of functionally graded coatings composed of suspension plasma sprayed hydroxyapatite and bioactive glass. J Biomed Mater Res B Appl Biomater. 2014;102(3):551-560.[25]Scislowska-Czarnecka A, Menaszek E, Szaraniec B, et al. Ceramic modifications of porous titanium: effects on macrophage activation. Tissue Cell. 2012;44(6):391-400. [26]陈炳鹏,王卓鹏,柳菁菁,等. 分子筛在医学领域的应用及作用机制[J]. 高等学校化学学报, 2011, 32(3):485-493.[27]Bedi RS, Chow G, Wang J, et al. Bioactive Materials for Regenerative Medicine: Zeolite-Hydroxyapatite Bone Mimetic Coatings. Advanced Engineering Materials. 2012; 14(3):200-206.[28]Bedi RS, Beving DE, Zanello LP, et al. Biocompatibility of corrosion-resistant zeolite coatings for titanium alloy biomedical implants. Acta Biomater. 2009;5(8):3265-3271.[29]Bedi RS, Zanello LP, Yan Y. Osteoconductive and Osteoinductive Properties of Zeolite MFI Coatings on Titanium Alloys. Advanced Functional Materials. 2009; 19(24):3856-3861.[30]Li Y, Jiao Y, Li X, et al. Improving the osteointegration of Ti6Al4V by zeolite MFI coating. Biochem Biophys Res Commun. 2015;460(2):151-156.[31]Thompson GJ, Puleo DA. Ti-6Al-4V ion solution inhibition of osteogenic cell phenotype as a function of differentiation timecourse in vitro. Biomaterials. 1996;17(20):1949-1954.[32]Hallab NJ, Vermes C, Messina C, et al. Concentration- and composition-dependent effects of metal ions on human MG-63 osteoblasts. J Biomed Mater Res. 2002;60(3):420-433.[33]Sun ZL, Wataha JC, Hanks CT. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J Biomed Mater Res. 1997;34(1):29-37.[34]Hu Y, Cai K, Luo Z, et al. Regulation of the differentiation of mesenchymal stem cells in vitro and osteogenesis in vivo by microenvironmental modification of titanium alloy surfaces. Biomaterials. 2012;33(13):3515-3528.[35]Zhang W, Wang G, Liu Y, et al. The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration.Biomaterials. 2013;34(13):3184-3195.[36]Yang HW, Lin MH, Xu YZ, et al. Osteogenesis of bone marrow mesenchymal stem cells on strontium-substituted nano-hydroxyapatite coated roughened titanium surfaces. Int J Clin Exp Med. 2015;8(1):257-264.[37]Li WR, Xie XB, Shi QS, et al. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol. 2010;85(4):1115-1122.[38]Wang J, Wang Z, Guo S, et al. Antibacterial and anti-adhesive zeolite coatings on titanium alloy surface. Microporous and Mesoporous Materials. 2011; 146(1-3):216-222.[39]Aryal S, Matsunaga K, Ching WY.Ab initio simulation of elastic and mechanical properties of Zn- and Mg-doped hydroxyapatite (HAP). J Mech Behav Biomed Mater. 2015;47:135-146.[40]Yoo JJ, Park YJ, Rhee SH, et al. Synthetic peptide-conjugated titanium alloy for enhanced bone formation in vivo. Connect Tissue Res. 2012;53(5):359-365.[41]Gu Z, Zhang X, Li L, et al. Acceleration of segmental bone regeneration in a rabbit model by strontium-doped calcium polyphosphate scaffold through stimulating VEGF and bFGF secretion from osteoblasts. Mater Sci Eng C Mater Biol Appl. 2013;33(1):274-281.[42]Xia Z, Yu X, Wei M. Biomimetic collagen/apatite coating formation on Ti6Al4V substrates. J Biomed Mater Res B Appl Biomater. 2012;100(3):871-881. |
| [1] | Xue Yadong, Zhou Xinshe, Pei Lijia, Meng Fanyu, Li Jian, Wang Jinzi . Reconstruction of Paprosky III type acetabular defect by autogenous iliac bone block combined with titanium plate: providing a strong initial fixation for the prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1424-1428. |
| [2] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [3] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [4] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [5] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [6] | Hu Weifan, Zheng Li, Li Dadi, Sun Yang, Zhao Fengchao. Overexpression of miR-25 downregulates titanium particle-induced osteoclast differentiation through the NFATc1 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 682-687. |
| [7] | Qiu Peng, Fu Qilin, Liu Min, Lan Yuyan, Wang Pin. Comparison of oral micro-adhesion on polyetheretherketone, zirconium dioxide, and pure titanium abutment [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 540-545. |
| [8] | Chen Shuo, Xiao Dongqin, Li Xingping, Ran Bin, Shi Feng, Zhang Chengdong, Deng Li, Huang Nanxiang, Liu Kang, Feng Gang, Duan Ke. Preparation and characterization of tantalum functional coating on titanium implant [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 546-552. |
| [9] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [10] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [11] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [12] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [13] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [14] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [15] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||