Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (38): 5650-5656.doi: 10.3969/j.issn.2095-4344.2016.38.004
Previous Articles Next Articles
Biological safety of silk fibroin/nano-hydroxyapatite composites
- 1Orthopedics Center of PLA, the 175th Hospital of PLA/Dongnan Hospital of Xiamen University, Zhangzhou 363000, Fujian Province, China; 2Department of Orthopedics, Changzheng Hospital, Second Military Medical University, Shanghai 200001, China
-
Received:2016-07-05Online:2016-09-16Published:2016-09-16 -
Contact:Ye Xiao-jian, M.D., Chief physician, Professor, Doctoral supervisor, Department of Orthopedics, Changzheng Hospital, Second Military Medical University, Shanghai 200001, China -
About author:Fan Chun-quan, M.D., Attending physician, Orthopedics Center of PLA, the 175th Hospital of PLA/Dongnan Hospital of Xiamen University, Zhangzhou 363000, Fujian Province, China -
Supported by:the National Natural Science Foundation of China, No. 81472071; the Medical Incubation Program for the Youth in PLA, No. 15QNP022; the Medical Innovation Project of the Nanjing Military Region of Chinese PLA, No. 15MS109
CLC Number:
Cite this article
Fan Chun-quan, Ye Xiao-jian.
share this article
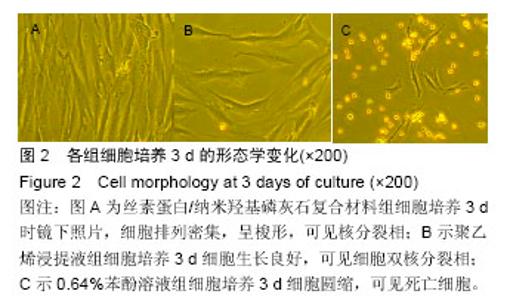
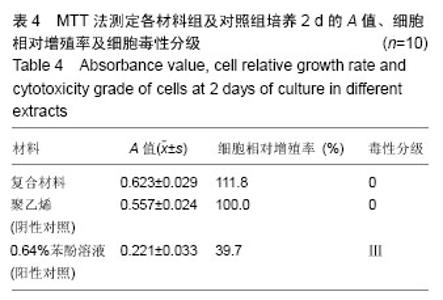
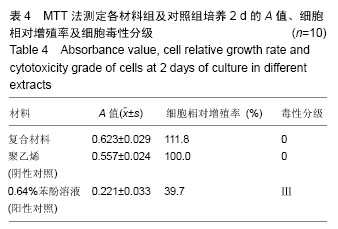
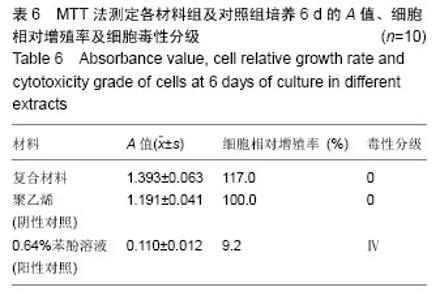
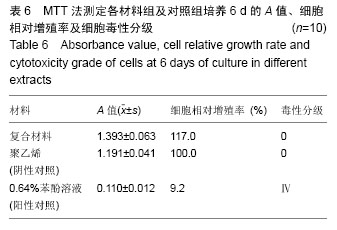
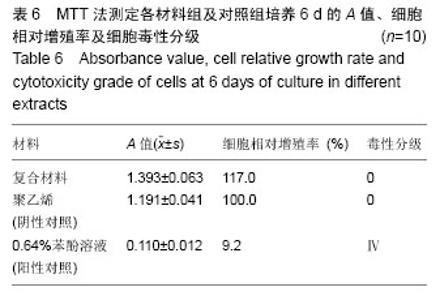
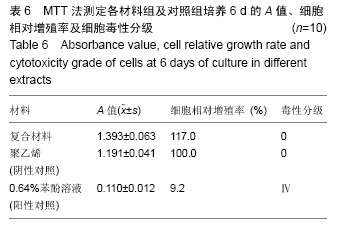
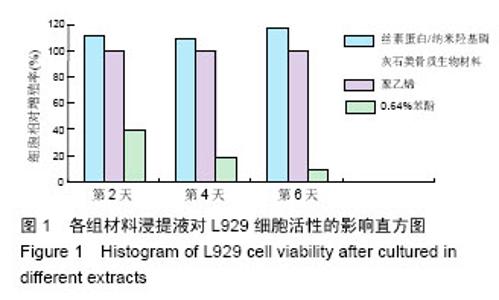
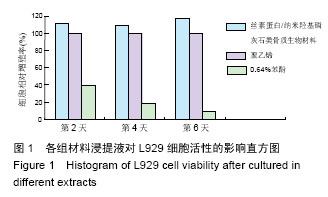
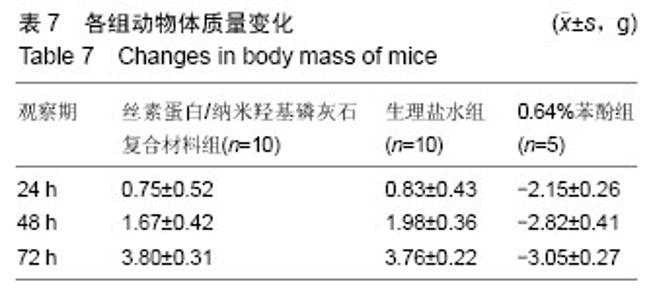
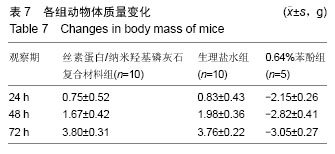
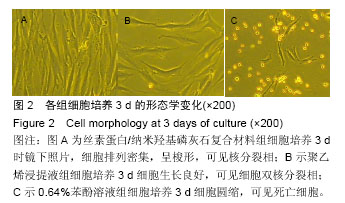
2.1.2 L929细胞与不同材料接触后的细胞黏附情况及形态学变化 在丝素蛋白/纳米羟基磷灰石复合材料浸提液组和阴性聚乙烯浸提液组细胞培养8 h后可以看见细胞形态基本正常,呈梭形或不规则三角形,细胞开始贴壁生长,随着时间延长,细胞数目增多,细胞突起伸展,出现分裂细胞。0.64%苯酚液阳性组在培养24 h时可见少量椭圆形细胞贴壁,大多数细胞悬浮。 培养3 d时可见丝素蛋白/纳米羟基磷灰石复合材料浸提液组(图2A)和聚乙烯浸提液组(图2B)细胞形态呈梭形或不规则三角形,细胞贴壁生长良好,排列密集规则,可见分裂细胞。培养3 d时在0.64%苯酚液组出现大部分细胞圆缩(图2C),死亡细胞明显增多,细胞基本不贴壁。因此通过细胞形态学观察,可以判定,丝素蛋白/纳米羟基磷灰石复合材料浸提液组和聚乙烯组浸提液无细胞毒性,0.64%苯酚液组具有重度细胞毒性。"
| [1]Du C, Cui FZ, Zhang W, et al. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J Biomed Mater Res. 2000;50(4): 518-527. [2]Kikuchi M, Itoh S, Ichinose S, et al. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials. 2001;22(13): 1705-1711. [3]Mann S. Molecular tectonics in biomineralization and biomimetic materials chemistry. Nature. 1993;365: 6. [4]Stupp SI, Braun PV. Molecular manipulation of microstructures: biomaterials, ceramics, and semiconductors. Science. 1997; 277(5330): 1242-1248. [5]Muthukumar M, Ober CK, Thomas EL. Competing Interactions and Levels of Ordering in Self-Organizing Polymeric Materials. Science. 1997; 277(5330): 1225-1232. [6]Chang MC, Ko CC, Douglas WH. Preparation of hydroxyapatite-gelatin nanocomposite. Biomaterials. 2003;24(17): 2853-2862. [7]Kasuga T, Ota Y, Nogami M, et al. Preparation and mechanical properties of polylactic acid composites containing hydroxyapatite fibers. Biomaterials. 2001; 22(1): 19-23. [8]Zhang JC, Lu HY, Lv GY, et al. The repair of critical-size defects with porous hydroxyapatite/ polyamide nanocomposite: an experimental study in rabbit mandibles. Int J Oral Maxillofac Surg. 2010; 39(5): 469-477. [9]Li Y, Cai YR, Kong XD, et al. Anisotropic growth of hydroxyapatite on the silk fibroin films. Appl Surf Sci. 2008;255(5, Part 1): 1681-1685. [10]Kundu B, Rajkhowa R, Kundu SC, et al. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65(4): 457-470. [11]Kuboyama N, Kiba H, Arai K, et al. Silk fibroin-based scaffolds for bone regeneration. J Biomed Mater Res B Appl Biomater. 2013;101(2): 295-302. [12]Zhu B, Li W, Lewis RV, et al. E-spun composite fibers of collagen and dragline silk protein: fiber mechanics, biocompatibility, and application in stem cell differentiation. Biomacromolecules. 2015;16(1): 202-213. [13]Jiang J, Hao W, Li Y, et al. Hydroxyapatite/regenerated silk fibroin scaffold-enhanced osteoinductivity and osteoconductivity of bone marrow-derived mesenchymal stromal cells. Biotechnol Lett. 2013; 35(4): 657-661. [14]Meinel L, Hofmann S, Karageorgiou V, et al. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26(2): 147-155. [15]Du CL, Jin J, Li YC, et al. Novel silk fibroin/ hydroxyapatite composite films: Structure and properties. Mater Sci Eng C. 2009;29(1): 62-68. [16]Korematsu A, Furuzono T, Yasuda S, et al. Nano-scaled hydroxyapatite/polymer composite III. Coating of sintered hydroxyapatite particles on poly(4-methacryloyloxyethyl trimellitate anhydride)- grafted silk fibroin fibers. J Mater Sci Mater Med. 2005; 16(1): 67-71. [17]You R, Li X, Luo Z, et al. Directional cell elongation through filopodia-steered lamellipodial extension on patterned silk fibroin films. Biointerphases. 2015;10(1): 011005. [18]Mobini S, Hoyer B, Solati-Hashjin M, et al. Fabrication and characterization of regenerated silk scaffolds reinforced with natural silk fibers for bone tissue engineering. J Biomed Mater Res A. 2013;101(8): 2392-2404. [19]Park HJ, Lee OJ, Lee MC, et al. Fabrication of 3D porous silk scaffolds by particulate (salt/sucrose) leaching for bone tissue reconstruction. Int J Biol Macromol. 2015;78: 215-223. [20]Pa?cu EI, Stokes J, McGuinness GB. Electrospun composites of PHBV, silk fibroin and nano-hydroxyapatite for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2013; 33(8): 4905-4916. [21]Uebersax L, Apfel T, Nuss KM, et al. Biocompatibility and osteoconduction of macroporous silk fibroin implants in cortical defects in sheep. Eur J Pharm Biopharm. 2013;85(1): 107-118. [22]Qi XN, Mou ZL, Zhang J, et al. Preparation of chitosan/silk fibroin/hydroxyapatite porous scaffold and its characteristics in comparison to bi-component scaffolds. J Biomed Mater Res A. 2014;102(2): 366-372. [23]Abdel-Fattah WI, Sallam AS, Diab AM, et al. Tailoring the properties and functions of phosphate/silk/Ag/ chitosan scaffolds. Mater Sci Eng C Mater Biol Appl. 2015;54: 158-168. [24]Fan CQ, Xu GH, He HL, et al. Facile fabrication of nano-hydroxyapatite/silk fibroin composite via a simplified coprecipitation route. J Mat Sci. 2010;45(21): 5. [25]Williams D. Objectivity in the evaluation of biological safety of medical devices and biomaterials. Med Device Technol. 1991;2(1): 44-48. [26]韩宁波, 赵建宁. 软骨组织工程常用支架制备技术[J]. 中国组织工程研究与临床康复, 2008,12(19): 3693-3696. [27]Richardson RR Jr, Miller JA, Reichert WM. Polyimides as biomaterials: preliminary biocompatibility testing. Biomaterials. 1993;14(8): 627-635. [28]Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2): 55-63. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||