Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (28): 4109-4116.doi: 10.3969/j.issn.2095-4344.2016.28.001
miR-106a regulates the osteogenic differentiation of human bone marrow mesenchymal stem cells by targeting bone morphogenetic protein receptor 2
Gu Su
- Third Ward, Department of Orthopedics, Nanyang City Central Hospital, Nanyang 473003, Henan Province, China
-
Received:2016-05-13Online:2016-07-01Published:2016-07-01 -
About author:Gu Su, Master, Attending physician, Third Ward, Department of Orthopedics, Nanyang City Central Hospital, Nanyang 473003, Henan Province, China
CLC Number:
Cite this article
Gu Su. miR-106a regulates the osteogenic differentiation of human bone marrow mesenchymal stem cells by targeting bone morphogenetic protein receptor 2[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(28): 4109-4116.
share this article
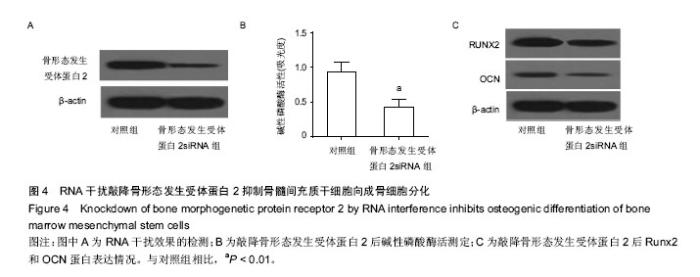
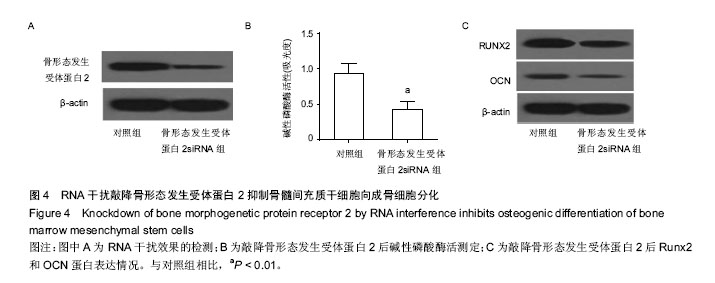
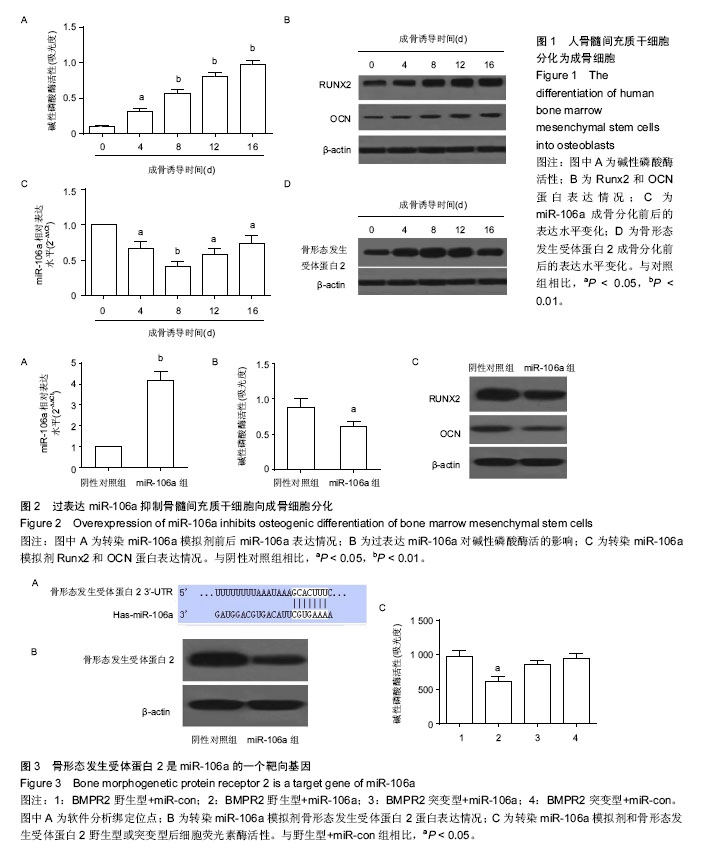
2.1 人骨髓间充质干细胞成功分化为成骨细胞 Runx2和OCN是成骨分化的标志蛋白,在成骨分化过程中,碱性磷酸酶活性上升,Runx2和OCN表达上升[27]。通过检测细胞Runx2和OCN蛋白的表达水平和碱性磷酸酶活性可以判断间充质干细胞成骨分化的进程。作者将人骨髓间充质干细胞使用成骨诱导培养基诱导分化,试剂盒检测碱性磷酸酶活性,western blot检测Runx2,OCN及骨形态发生受体蛋白2表达,real-time PCR检测分化前后miR-106a表达。结果表明,随着分化诱导时间的增加,碱性磷酸酶活性逐渐上升,成骨分化标志蛋白Runx2和OCN的表达也不断上升,说明在实验过程中人骨髓间充质干细胞被成功诱导为成骨细胞。随着成骨分化的进行,miR-106a表达逐渐下降,暗示miR-106a在成骨分化过程中有负调控作用。骨形态发生受体蛋白2表达逐渐升高,在第8天达到高峰,和miR-106a的变化趋势相反(图1)。这些结果暗示在骨髓间充质干细胞成骨分化的过程中,miR-106a和骨形态发生受体蛋白2的表达可能存在负相关性。 2.2 miR-106a抑制骨髓间充质干细胞向成骨细胞分化 为了探索miR-106a在成骨分化过程中的作用,作者将miR-106a模拟剂转染至骨髓间充质干细胞,检测转染miR-106a模拟剂对分化指标的影响。结果表明转染miR-106a模拟剂后,miR-106a表达量上升,碱性磷酸酶活性下降,成骨分化标志蛋白Runx2和OCN表达量下降(图2),表明miR-106a模拟剂抑制了骨髓间充质干细胞向成骨细胞的分化。 2.3 miR-106a靶向骨形态发生受体蛋白2抑制骨髓间充质干细胞向成骨细胞分化 使用Target Scan软件在线查找miR-106a的靶基因,结果发现,骨形态发生受体蛋白2是miR-106a的潜在靶基因。图3A是Target Scan预测的骨形态发生受体蛋白2和miR-106a的绑定位点。为了检测miR-106a能否调控骨形态发生受体蛋白2表达,作者使用Western blot检测了转染miR-106a模拟剂后骨形态发生受体蛋白2蛋白表达情况。结果发现转染miR-106a模拟剂后,骨形态发生受体蛋白2蛋白表达下降(图3B)。使用荧光素酶报告系统,共转染miR-106a和骨形态发生受体蛋白2 3’-UTR野生型或骨形态发生受体蛋白23’-UTR突变型,转染36 h后收集细胞测定荧光素酶活性。结果发现,和转染miR-106a阴性对照和骨形态发生受体蛋白2 3’-UTR野生型的细胞相比,共转染miR-106a和骨形态发生受体蛋白2 3’-UTR野生型的细胞荧光素酶活性显著下降,miR-106a模拟剂对骨形态发生受体蛋白2 3’-UTR突变型荧光素酶活性没有抑制作用。这些结果表明miR-106a可以抑制骨形态发生受体蛋白2 3’-UTR的转录活性,从而调控骨形态发生受体蛋白2的表达(图3)。 2.4 敲降骨形态发生受体蛋白2抑制骨髓间充质干细胞向成骨细胞分化 为了研究骨形态发生受体蛋白2在骨髓间充质干细胞成骨分化中的作用,作者使用RNA干扰技术敲降骨形态发生受体蛋白2,诱导成骨分化并检测成骨分化相关指标。如图4A所示,转染骨形态发生受体蛋白2 siRNA的细胞骨形态发生受体蛋白2表达量下降了80%,干扰效果较好。敲降骨形态发生受体蛋白2表达和过表达miR-106a的结果类似,碱性磷酸酶活性下降(图4B),成骨分化标志蛋白Runx2和OCN的表达下降(图4C)。这些结果表明敲降骨形态发生受体蛋白2表达,人骨髓间充质干细胞成骨分化受到抑制。"
| [1] Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8(3):147-159. [2] Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19(8):458-466. [3] Vimalraj S, Selvamurugan N. MicroRNAs: Synthesis, Gene Regulation and Osteoblast Differentiation. Curr Issues Mol Biol. 2013;15:7-18. [4] Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324-336. [5] Vimalraj S, Selvamurugan N. MicroRNAs expression and their regulatory networks during mesenchymal stem cells differentiation toward osteoblasts. Int J Biol Macromol. 2014;66:194-202. [6] Wei J, Li H, Wang S, et al. let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev. 2014; 23(13):1452-1463. [7] Lian JB, Stein GS, van Wijnen AJ, et al. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8(4):212-227. [8] Taipaleenmäki H, Bjerre Hokland L, Chen L, et al. Mechanisms in endocrinology: micro-RNAs: targets for enhancing osteoblast differentiation and bone formation. Eur J Endocrinol. 2012;166(3):359-371. [9] Thomas M, Lieberman J, Lal A. Desperately seeking microRNA targets. Nat Struct Mol Biol. 2010;17(10): 1169-1174. [10] Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861-874. [11] Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175-205. [12] Schoolmeesters A, Eklund T, Leake D, et al. Functional profiling reveals critical role for miRNA in differentiation of human mesenchymal stem cells. PLoS One. 2009; 4(5):e5605. [13] Baglìo SR, Devescovi V, Granchi D, et al. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene. 2013;527(1):321-331. [14] Hu R, Li H, Liu W, et al. Targeting miRNAs in osteoblast differentiation and bone formation. Expert Opin Ther Targets. 2010;14(10):1109-1120. [15] Luzi E, Marini F, Sala SC, et al. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res. 2008; 23(2):287-295. [16] Kim YJ, Bae SW, Yu SS, et al. miR-196a regulates proliferation and osteogenic differentiation in mesenchymal stem cells derived from human adipose tissue. J Bone Miner Res. 2009;24(5):816-825. [17] Huang S, Wang S, Bian C, et al. Upregulation of miR-22 promotes osteogenic differentiation and inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by repressing HDAC6 protein expression. Stem Cells Dev. 2012; 21(13): 2531-2540. [18] Zhu M, Zhang N, He S, et al. MicroRNA-106a targets TIMP2 to regulate invasion and metastasis of gastric cancer. FEBS Lett. 2014;588(4):600-607. [19] Yuan R, Zhi Q, Zhao H, et al. Upregulated expression of miR-106a by DNA hypomethylation plays an oncogenic role in hepatocellular carcinoma. Tumour Biol. 2015;36(4):3093-3100. [20] Xie X, Liu HT, Mei J, et al. miR-106a promotes growth and metastasis of non-small cell lung cancer by targeting PTEN. Int J Clin Exp Pathol. 2015;8(4): 3827-3834. [21] Li P, Xu Q, Zhang D, et al. Upregulated miR-106a plays an oncogenic role in pancreatic cancer. FEBS Lett. 2014;588(5):705-712. [22] Wang Z, Liu M, Zhu H, et al. miR-106a is frequently upregulated in gastric cancer and inhibits the extrinsic apoptotic pathway by targeting FAS. Mol Carcinog. 2013;52(8):634-646. [23] Wang W, Corrigan-Cummins M, Hudson J, et al. MicroRNA profiling of follicular lymphoma identifies microRNAs related to cell proliferation and tumor response. Haematologica. 2012;97(4):586-594. [24] Zhi F, Zhou G, Shao N, et al. miR-106a-5p inhibits the proliferation and migration of astrocytoma cells and promotes apoptosis by targeting FASTK. PLoS One. 2013;8(8):e72390. [25] Gao J, Yang T, Han J, et al. MicroRNA expression during osteogenic differentiation of human multipotent mesenchymal stromal cells from bone marrow. J Cell Biochem. 2011;112(7):1844-1856. [26] 张贤,蔡建平,张艳红,等.杜仲诱导大鼠间充质干细胞成骨分化中成骨与成脂相关转录因子的表达[J].中国组织工程研究,2010,14(19):3523-3526. [27] Sun H, Ye F, Wang J, et al. The upregulation of osteoblast marker genes in mesenchymal stem cells prove the osteoinductivity of hydroxyapatite/tricalcium phosphate biomaterial. Transplant Proc. 2008;40(8): 2645-2648. [28] Nukavarapu SP, Dorcemus DL. Osteochondral tissue engineering: current strategies and challenges. Biotechnol Adv. 2013;31(5):706-721. [29] Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133(2):217-222. [30] Wang Z, Wang B, Shi Y, et al. Oncogenic miR-20a and miR-106a enhance the invasiveness of human glioma stem cells by directly targeting TIMP-2. Oncogene. 2015;34(11):1407-1419. [31] Larabee SM, Coia H, Jones S, et al. miRNA-17 members that target Bmpr2 influence signaling mechanisms important for embryonic stem cell differentiation in vitro and gastrulation in embryos. Stem Cells Dev. 2015;24(3):354-371. [32] Selbach M, Schwanhäusser B, Thierfelder N, et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58-63. [33] 罗渊,田亚平.microRNA 的调控和被调控[J].中国生物化学与分子生物学报,2014,30(12):1176-1181. [34] Wei Y, Nazari-Jahantigh M, Chan L, et al. The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155- dependent pathway during atherosclerosis. Circulation. 2013;127(15):1609-1619. [35] Zeng Y, Qu X, Li H, et al. MicroRNA-100 regulates osteogenic differentiation of human adipose-derived mesenchymal stem cells by targeting BMPR2. FEBS Lett. 2012;586(16):2375-2381. [36] Yang Z, Hao J, Hu ZM. MicroRNA expression profiles in human adipose-derived stem cells during chondrogenic differentiation. Int J Mol Med. 2015; 35(3):579-586. [37] Yuan B, Sun GJ, Zhang GL, et al. Identification of target genes for adenohypophysis-prefer miR-7 and miR-375 in cattle. Genet Mol Res. 2015;14(3):9753- 9763. [38] Brock M, Trenkmann M, Gay RE, et al. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3- microRNA cluster 17/92 pathway. Circ Res. 2009;104 (10):1184-1191. [39] Chen F, Lin X, Xu P, et al. Nuclear Export of Smads by RanBP3L Regulates Bone Morphogenetic Protein Signaling and Mesenchymal Stem Cell Differentiation. Mol Cell Biol. 2015;35(10):1700-1711. [40] Li B, Tian XB, Hu RY, et al. Mechanism of BMP and TG2 in mesenchymal stem cell osteogenesis. Eur Rev Med Pharmacol Sci. 2015;19(22):4214-4219. [41] Wang A, Ding X, Sheng S, et al. Bone morphogenetic protein receptor in the osteogenic differentiation of rat bone marrow stromal cells. Yonsei Med J. 2010;51(5): 740-745. [42] 王茸影,易静.骨形成蛋白调控成骨分化的信号机制[J].生命科学,2005,17(1):34-39. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [3] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [4] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [5] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [6] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [7] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [8] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [9] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [10] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [11] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [12] | Zhou Wu, Wang Binping, Wang Yawen, Cheng Yanan, Huang Xieshan. Transforming growth factor beta combined with bone morphogenetic protein-2 induces the proliferation and differentiation of mouse MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3630-3635. |
| [13] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [14] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [15] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||