Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (19): 2864-2871.doi: 10.3969/j.issn.2095-4344.2016.19.019
Previous Articles Next Articles
Effects of mechanical factors on proliferation and differentiation of tissue- engineered stem cells
Jiang Dong1, Wang Ji-hong2
- 1Inner Mongolia Medical University, Hohhot 010030, Inner Mongolia Autonomous Region, China
2Department of Hand-Foot Microsurgery, Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010030, Inner Mongolia Autonomous Region, China
-
Received:2016-03-15Online:2016-05-06Published:2016-05-06 -
Contact:Wang Ji-hong, M.D., Chief physician, Department of Hand-Foot Microsurgery, Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010030, Inner Mongolia Autonomous Region, China -
About author:Jiang Dong, Inner Mongolia Medical University, Hohhot 010030, Inner Mongolia Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 81441117
CLC Number:
Cite this article
Jiang Dong, Wang Ji-hong. Effects of mechanical factors on proliferation and differentiation of tissue- engineered stem cells[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(19): 2864-2871.
share this article
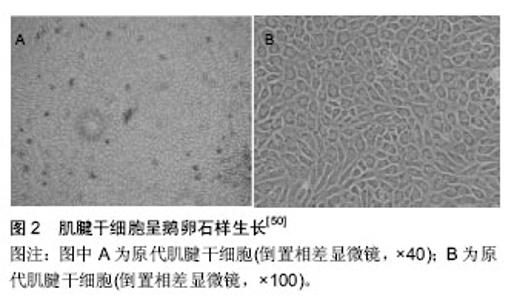
2.1 培养组织工程肌腱的干细胞 主要包括间充质干细胞、肌腱干细胞[1-3]、胚胎干细胞。 2.1.1 间充质干细胞 Cohnheim等[4-5]在140多年前发现骨髓中存在非造血干细胞,并且能分化为成纤维细胞和许多中胚层起源的细胞。间充质干细胞是在19世纪60到70年代首次提出的,Friedenstein等[6]和他的团队在骨髓细胞培养时发现有一些细长的细胞不具备造血功能,却具有个体细胞增长的能力。间充质干细胞最初被人们定义为从成人骨髓中分离和培养的多功能干细胞,然而根据干细胞的细胞特征及研究方向其命名略有不同。在适当的条件下,间充质干细胞在体外可以分化为几个谱系的细胞,如成骨细胞[7]、软骨细胞[8]、脂肪细胞[9]、肌细胞[10]、神经细胞、胰腺和肝细胞谱系等。间充质干细胞在体内分布广泛,取材较为方便,在骨髓、脂肪[11-12]、筋膜和骨骼肌组织中即可分离得到[13]。骨髓间充质干细胞的表面抗原表达谱研究详尽,但是变异仍然存在于骨髓间充质干细胞种群,于是2006年国际细胞治疗协会(The International Society for Cellular Therapy)做出规定[14]:骨髓间充质干细胞表面特异性抗原必须是CD105(+),CD73(+),CD90(+),CD45(-),CD34(-),CD18(+),CD14/CD11b(-),CD79a/CD19(-),这使其分选和纯化有了国际认同的统一标准。目前国际上普遍的分离间充质干细胞的方法包括:组织消化法、密度梯度离心法、免疫磁珠法和全骨髓贴壁法,前者应用较多。间充质干细胞具有诸多优点,首先是组织损伤修复作用,其可以在炎症递质的引导下迁移到受损伤部位参与组织修复[15],但在临床应用方面还有许多的障碍,主要有细胞工程技术的不成熟、费用昂贵、引入体内的安全性问题等,克服这些困难,还需要了解分子和细胞在生理和病理状态时的机制,如胚胎发育、炎症、伤口愈合、癌症转移等。解决这些细胞机制问题,将对干细胞的研究具有更强的导向性和效用性,并将取代某些不可治愈疾病当前的临床治疗方法。其次间充质干细胞是较原始的干细胞,免疫原性较低甚至可以忽略,这就削弱器官移植的配型障碍和增加移植物存活的机会[16],为临床治疗提供了另一种优质的疗法[17-18]。 2.1.2 肌腱干细胞 传统的学术观念认为,肌腱仅由散在分布梭形腱细胞和纵行排列的胶原纤维组成。2007年de Mos等[19]研究表明,肌腱细胞中存在一种具有多分化潜能、无限增殖的细胞。此后在研究人类和小鼠的肌腱细胞时,发现其拥有自我复制、更新、并向来自于同胚层组织细胞分化的能力,具有干细胞的特性,如具有克隆传代、多分化潜质和自我更新能力,在组织工程肌腱的研究中也是比较理想的种子细胞。目前研究主要集中在肌腱干细胞的提取、体外培养及分化(三系分化能力即成骨、软骨和脂肪的能力)。肌腱干细胞表面抗原标记物阳性表达的有[20-22]:Sca-1和SSEA-4,CD29,CD44,CD90,CD105,STRO-1,CD146,Nucleostemin,Oct-4,CD14, CD31,CD34,CD18,CD117,CD45,Flk-1,CD144和CD106,不同物种来源的肌腱干细胞表面标记物略有不同。肌腱干细胞属于成体干细胞、单能干细胞,可从哺乳动物肌腱组织中获得[23-25],因此体外培养时组织细胞的来源广泛、分化调控相对简单、特定组织分化能力强。当下其广泛应用还存在很多难题,首先肌腱干细胞虽然来源广泛,但在组织中的含量过少,因此相关技术能力要求较高;其次虽然肌腱干细胞在腱组织受损时起主导的修复作用,但在健康的机体组织中其处于静止状态,只有当损伤因子打破细胞内外环境平衡,在神经和体液的调节下,肌腱干细胞苏醒并开始增殖和分化,以修复受损的腱组织。体外培养时,如何更有效的激活肌腱干细胞来缩短周期降低成本,需要研究者的不断努力。 2.1.3 胚胎干细胞 1981年Evans和Kaufman在怀孕小鼠的胎盘中首次分离得到小鼠的胚胎干细胞[26]。随后Martin以免疫外科法从鼠囊胚滋养层得到胚胎干细胞[27]。Brook等[28]进一步完善了小鼠胚胎干细胞的分离方法,以致从许多品系小鼠包括近交系和突变系,均可获得胚胎干细胞。1998年Thomson等[29]在小鼠胚胎干细胞研究基础上成功用女性囊胚组织建立人胚胎干细胞系。目前国内外研究人员已从仓鼠、大鼠[30]、兔[31]、猪、牛[32]、山羊等分离获得了胚胎干细胞,胚胎干细胞具有多能性,特点是可以通过细胞分化发育成为外胚层、中胚层及内胚层3种胚层的组织细胞,对胚胎的发育和分化起着至关重要的作用[33]。已有研究证明胚胎干细胞可以分化为生命体的所有细胞,包括前面提到的间充质干细胞、肌腱干细胞及其他一些具有特定属性的干细胞等[34]。胚胎干细胞较其他干细胞具有最广泛和原始的分化潜能。在目前的研究中已发现其胞浆中的甲胎蛋白和TLMA活性较高,而且在未分化成熟的胚胎干细胞中Oct3/4、TDGF、Sox2、LeftyA、Thy-1、FGF4、Rex1、SSEA3、SSEA4、TRA-1-81、TRA-2-54 和 GCTM-2高表达,这些为胚胎干细胞分离和纯化提供准确的标记。但是胚胎干细胞存在伦理道德方面的争议,另外由于其具有全能分化潜力,在进行损伤组织修复时会由于细胞内环境的紊乱,激活它的病理性增殖、分化潜能,脱离机体监控,进而成瘤或致畸。Maya-Espinosa等[35]在研究大鼠时发现,胚胎干细胞会修复因脑卒中受损的纹状体,促进神经元的分化,这为人类神经细胞的修复提供重要的基础。Khan等[36]在研究小鼠胚胎干细胞时发现,其具有促进心肌细胞再生和调节心肌细胞分化的能力进而促进心肌修复。 除上述3种细胞外,成纤维细胞、角质细胞、上皮细胞、成骨细胞等均可以成为“再生肌腱”的种子细胞。这些细胞虽然有来源广泛、伦理限制小、致畸胎率低等优点,但它们属于成体已分化的细胞,再分化能力不足;其次培养出的组织容易属性混乱及不可避免的免疫原性,因此需要更高要求的组织工程细胞培养技术。 2.2 力学因素及组织工程机械应力分类 2.2.1 诱导分化因素简述 细胞在体外培养过程中受到会诸多因素的影响,例如生长因子、体液离子、体外冲击波、热休克、机械应力等,不同因素会诱导其向不同方向分化。由于人体肌肉系统随时随地都在做着物理运动,力学刺激充斥着细胞生存的微环境。因此机械力学刺激是调节“细胞再生肌腱”比较重要的一环。 2.2.2 应力系统分类 细胞培养过程中的微环境应力刺激,主要是通过外界力学装置传导系统施加。目前广泛应用的力学系统主要有:①细胞流体切应力系统又称流体剪切应力系统。正常机体在运动过程中,组织液受到重力、渗透压力、肌肉及其他组织牵拉、收缩的压力,导致组织液在细胞周围不规则流动使细胞受力。根据这一流变原理将细胞培养在多通道循环流动小室中,以尽量模拟细胞在体内受切应力的微环境。目前国内外常见的细胞流体切应力模型有:STR-4000多通道微流控流体切应力加载模型和Flexflow流体切应力同时抻拉细胞的平行板流室模型。②静止应压力系统,细胞的生活空间某种程度上可以认为是相对封闭的,因此在外力作用下细胞表面会均匀受到体液的正性压力或负性压力。将细胞和组织培养在封闭的培养容器中,可以通过液体或气体对培养物施加压力,其优点是培养物受力均匀、不必与培养基粘连。目前常见的应压力系统主要是固静止压力系统和周期分段调节静止应压力系统。③牵张应力系统,肌肉收缩最直观的表现就是细胞受到牵拉,从而激活离子通道进行细胞信号的传导,从目前的文献可以看出此系统在细胞培养及分化过程中起着比较重要的作用。实验时将种子细胞接种在细胞支架上,再对支架施加温和的牵张应力,然后在培养基中培养。徐源[37]通过家兔的实验证实,在牵张应力系统培养下的再生肌腱生物结构及力学性能更接近生理状态下的肌腱。应力牵张系统主要有持续应力牵张系统和周期应力牵张系统。此外还有离心力培养系统和单个细胞微观吸吮技术。前者力学指标量化困难、力学刺激不易控制。后者操作过程过于复杂,技术性要求高。因此从实用性和科学性的角度看前3类系统更有应用的价值。 2.3 力学刺激对种子细胞的影响 1893年Julius Wolff在前人研究的基础上阐述到骨组织对力学刺激具有很强的适应性。随着刺激的性质和程度的不同,其内部结构及外部形态也会发生适应性并遵循数学规律的改变。Julius Wolff定律对后人关于相关领域研究有着里程碑式的意义[38]。后来Ilizarov发现给予组织持续温和应力刺激时会使某些组织细胞代谢活跃和返祖,再次分类及分化[39]。随着医学的发展这两大基本定律早已应用于组织工程。目前的研究成果可以得知:力学刺激对种子细胞的增殖和分化有促进和调节作用,其机制是一个复杂而精密的过程,需要在力学微环境下表达的、高度有序的细胞因子(第一信使、第二信使[40])参与细胞信号通路,调节基因的转录和表达[41],产生各种功能蛋白质调节生理进程[42]。 2.3.1 牵张应力对于种子细胞的影响 1992年Hynes等[43]在研究成体干细胞时证实:牵张应力是通过经典信号传导途径诱导种子细胞(成纤维细胞)增殖和分化,即细胞外基质-整合素-细胞骨架复合系统。在真核生物中细胞骨架系统包括微管、微丝、中间纤维、核基质、核纤层和细胞外基质。同样牵张应力也是通过上述传导通路最终影响间充质干细胞、肌腱干细胞、胚胎干细胞。Simmons等[44]实验时给间充质干细胞施加0.25 Hz、3%张力后细胞的增殖减慢,但3%的牵张力可以促进其向成骨细胞分化[45],10%的张力则会使其向平滑肌细胞分化,复杂张力则会使细胞向成骨细胞分化[46]。2007年有学者在研究大鼠间充质干细胞时发现[47]:当频率为1 Hz、8%张力、持续60 min时对间充质干细胞具有最强的增殖作用。黎润光等[48]给人间充质干细胞施以张力时发现:在48 h前12%的张力促进细胞增殖的效果更强,18%的张力则会抑制细胞的增殖,但在48 h之后随着时间的增加抑制作用会成为主要的矛盾。后来学者发现细胞内的F-肌动蛋白[49],在初始力学微环境下会变得十分活跃,但随着时间的延长其会失活。杨广华等[50]在研究小鼠肌腱干细胞(图2)时发现:0.5,1,3 Hz的牵张频率对于其增殖与未受力组相比基本相同,但2 Hz却促进肌腱干细胞增殖。有研究表明在4%应变力的作用下肌腱干细胞向肌腱组织分化,当应变力8%时,肌腱干细胞既可以向肌腱组织分化又可以向脂肪组织、软骨组织及骨组织分化[51]。Shi等[52]通过实验发现2%的应力刺激可使其向成骨组织分化。胚胎干细胞体外培养时同样也会因张力频率及大小的不同,对增殖分化有一定的影响,在此不一一赘述。"
| [1] Rui YF, Lui PP, Li G, et al. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16(5):1549-1558. [2] Yin Z, Chen X, Chen JL, et al. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31(8):2163-2175. [3] Tan Q, Lui PP, Rui YF, et al. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A. 2012;18(7-8):840-851. [4] Cohnheim J. Ueber Entzündung und Eiterung. Path Anat Physiol Klin Med. 1867; 40(1-2):1-79. [5] Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5): 267-274. [6] Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381-390. [7] Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393-395. [8] Santos TS, Abuna RP, Castro Raucci LM, et al. Mesenchymal Stem Cells Repress Osteoblast Differentiation Under Osteogenic-Inducing Conditions. J Cell Biochem. 2015;116(12):2896-2902. [9] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [10] Klein G, Hart ML, Brinchmann JE, et al. Mesenchymal stromal cells for sphincter regeneration. Adv Drug Deliv Rev. 2015;82-83:123-136. [11] Yu J, Tu YK, Tang YB, et al. Stemness and transdifferentiation of adipose-derived stem cells using L-ascorbic acid 2-phosphate-induced cell sheet formation. Biomaterials. 2014;35(11):3516-3526. [12] Lin CY, Huang CH, Wu YK, et al. Maintenance of human adipose derived stem cell (hASC) differentiation capabilities using a 3D culture. Biotechnol Lett. 2014;36(7):1529-1537. [13] Sekiya N, Tobita K, Beckman S, et al. Muscle-derived stem cell sheets support pump function and prevent cardiac arrhythmias in a model of chronic myocardial infarction. Mol Ther. 2013;21(3):662-669. [14] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [15] Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. [16] Vanikar AV, Trivedi HL, Kumar A,et al. Mesenchymal stem cells and transplant tolerance. Nephrology (Carlton). 2014;19(7):369-374. [17] Lin CC, Fu SJ. Osteogenesis of human adipose-derived stem cells on poly(dopamine)-coated electrospun poly(lactic acid) fiber mats. Mater Sci Eng C Mater Biol Appl. 2016;58:254-263. [18] Zheng X, Wang W, Liu S, et al. Enhancement of chondrogenic differentiation of rabbit mesenchymal stem cells by oriented nanofiber yarn-collagen type I/hyaluronate hybrid. Mater Sci Eng C Mater Biol Appl. 2016;58:1071-1076. [19] de Mos M, Koevoet WJ, Jahr H, et al. Intrinsic differentiation potential of adolescent human tendon tissue: an in-vitro cell differentiation study. BMC Musculoskelet Disord. 2007;8:16. [20] 秦胜男,王文,傅世铨,等.人髌腱干细胞的分离培养与鉴定[J].中国矫形外科杂志,2014,22(24):2269-2276. [21] 胡超,唐康来,陈万,等.大鼠跟腱来源肌腱干细胞的分离培养及鉴定[J].第三军医大学学报,2013,35(11):1097-1101. [22] Zhou Z, Akinbiyi T, Xu L, et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010;9(5):911-915. [23] Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. [24] Rui YF, Lui PP, Li G, et al. Isolation and characterization of multipotent rat tendon-derived stem cells.Tissue Eng Part A. 2010;16(5):1549-1558. [25] Yin Z, Chen X, Chen JL, et al. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31(8):2163-2175. [26] Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981; 292(5819):154-156. [27] Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78(12):7634-7638. [28] Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci U S A. 1997;94(11):5709-5712. [29] Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145-1147. [30] Buehr M, Meek S, Blair K, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008; 135(7):1287-1298. [31] Moreadith RW, Graves KH. Derivation of pluripotential embryonic stem cells from the rabbit. Trans Assoc Am Physicians. 1992;105:197-203. [32] Mitalipova M, Beyhan Z, First NL. Pluripotency of bovine embryonic cell line derived from precompacting embryos. Cloning. 2001;3(2):59-67. [33] Yamashita A, Takada T, Omatsu-Kanbe M, et al. Monkey embryonic stem cells differentiate into adipocytes in vitro. Cloning Stem Cells. 2006;8(1):3-9. [34] Nakamura K, Aizawa K, Yamauchi J, et al. Hyperforin inhibits cell proliferation and differentiation in mouse embryonic stem cells. Cell Prolif. 2013;46(5):529-537. [35] Maya-Espinosa G, Collazo-Navarrete O, Millán-Aldaco D, et al. Mouse embryonic stem cell-derived cells reveal niches that support neuronal differentiation in the adult rat brain. Stem Cells. 2015;33(2):491-502. [36] Khan M, Nickoloff E, Abramova T, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117(1):52-64. [37] 徐源.周期张应力下TDSCs与(PLLA-CL)-Col支架构建组织工程肌腱的研究[D].重庆:第三军医大学,2014. [38] Woff J. The Law of Bone Remo deling. Berlin: Springer-Verlag Berlin Heidelberg. 1986. [39] Wang JH, Guo Q, Li B. Tendon biomechanics and mechanobiology-a minireview of basic concepts and recent advancements. J Hand Ther. 2012;25(2):133-140. [40] Sinlapabodin S, Amornsudthiwat P, Damrongsakkul S, et al. An axial distribution of seeding, proliferation, and osteogenic differentiation of MC3T3-E1 cells and rat bone marrow-derived mesenchymal stem cells across a 3D Thai silk fibroin/gelatin/hydroxyapatite scaffold in a perfusion bioreactor. Mater Sci Eng C Mater Biol Appl. 2016;58:960-970. [41] Killian ML, Cavinatto L, Galatz LM, et al. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228-237. [42] Cunha B, Aguiar T, Silva MM, et al. Exploring continuous and integrated strategies for the up- and downstream processing of human mesenchymal stem cells. J Biotechnol. 2015;213:97-108. [43] Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11-25. [44] Simmons CA, Matlis S, Thornton AJ, et al. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36(8):1087-1096. [45] Jang JY, Lee SW, Park SH, et al. Combined effects of surface morphology and mechanical straining magnitudes on the differentiation of mesenchymal stem cells without using biochemical reagents. J Biomed Biotechnol. 2011;2011:860652. [46] 王秋实,杨孝勤,朱晓文,等.动静态不同牵张条件下大鼠骨髓间充质干细胞的增殖与分化[J].中国组织工程研究, 2013,17(36):6396-6402. [47] Song G, Ju Y, Shen X, et al. Mechanical stretch promotes proliferation of rat bone marrow mesenchymal stem cells. Colloids Surf B Biointerfaces. 2007;58(2):271-277. [48] 黎润光,邵景范,魏明发,等.牵张应力对人骨髓间充质干细胞增殖及细胞周期的影响[J].中国组织工程研究与临床康复, 2007, 11(7):1247-1251. [49] 赵红斌,张西正,吴金辉,等.不同应变对骨髓间充质干细胞系细胞骨架影响的研究[J].激光生物学报, 2007, 16(1): 12-17. [50] 杨广华. 机械牵伸频率对肌腱干细胞增殖分化的影响[D].重庆:第三军医大学,2014. [51] Zhang J, Wang JH. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res. 2010; 28(5):639-643. [52] Shi Y, Fu Y, Tong W, et al. Uniaxial mechanical tension promoted osteogenic differentiation of rat tendon- derived stem cells (rTDSCs) via the Wnt5a-RhoA pathway. J Cell Biochem. 2012;113(10):3133-3142. [53] Nonaka S, Shiratori H, Saijoh Y, et al. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418(6893):96-99. [54] 邢宏力,边云飞武卫东,等.流体剪切力对5-氮杂胞苷诱导大鼠骨髓间充质干细胞向心肌样细胞分化的影响[J].中国动脉硬化杂志,2010,18(12):951-955. [55] Yamamoto K, Sokabe T, Watabe T, et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288(4): H1915-1924. [56] Kreke MR, Goldstein AS. Hydrodynamic shear stimulates osteocalcin expression but not proliferation of bone marrow stromal cells. Tissue Eng. 2004; 10(5-6):780-788. [57] Li YJ, Batra NN, You L, et al. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res. 2004;22(6):1283-1289. [58] Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36(6): 1047-1055. [59] Riddle RC, Taylor AF, Genetos DC, et al. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol Cell Physiol. 2006;290(3):C776-784. [60] Adamo L, Naveiras O, Wenzel PL, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459(7250):1131-1135. [61] 王秋实,杨孝勤,朱晓文,等.动静态不同牵张条件下大鼠骨髓间充质干的增殖与分化[J].中国组织工程研究,2013, 17(36):6396-6402. [62] Kobayashi N, Yasu T, Ueba H, et al. Mechanical stress promotes the expression of smooth muscle-like properties in marrow stromal cells. Exp Hematol. 2004; 32(12):1238-1245. [63] He J, Wu F, Wang D, et al. Modulation of cationicity of chitosan for tuning mesenchymal stem cell adhesion, proliferation, and differentiation. Biointerphases. 2015; 10(4):04A304. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Xu Feng, Kang Hui, Wei Tanjun, Xi Jintao. Biomechanical analysis of different fixation methods of pedicle screws for thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1313-1317. |
| [4] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [5] | Chen Xinmin, Li Wenbiao, Xiong Kaikai, Xiong Xiaoyan, Zheng Liqin, Li Musheng, Zheng Yongze, Lin Ziling. Type A3.3 femoral intertrochanteric fracture with augmented proximal femoral nail anti-rotation in the elderly: finite element analysis of the optimal amount of bone cement [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1404-1409. |
| [6] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [7] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [8] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [9] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [10] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [11] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [12] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [13] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [14] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [15] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||